新生儿暴露于七氟醚可抑制dhhc5介导的少突胶质细胞中TfR1棕榈酰化,导致髓鞘发育低下和神经损伤
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
新生儿麻醉相关的神经损伤是一个值得关注的问题,与少突胶质细胞功能障碍密切相关。然而,少突胶质细胞的持续发育(髓鞘形成)与麻醉暴露的短期易损性之间存在显著的时间差异。鉴于新生儿时期少突胶质细胞对铁的需求显著增加,我们的目的是阐明铁稳态的潜在作用和潜在机制,特别是关注转铁蛋白受体1 (TfR1)在控制麻醉的短暂易感性中的作用。方法从出生后(P)6 ~ P8天,给药七氟醚(3 %,2 h/d)。随后,对P8和/或P32进行行为测试、遗传调节、共免疫沉淀试验、酰基树脂辅助捕获试验和单细胞RNA测序。结果新生儿暴露于七氟醚后,观察到的认知障碍和P32骨髓化低可归因于铁积累和铁凋亡,特别是在胼胝体(CC)少突胶质细胞内。这种铁死亡是由瞬时表达的TfR1的内吞作用增强介导的,而不是其过度表达,这是由于棕榈酰化受到抑制。在21个棕榈酰转移酶中,只有Asp-His-His-Cys5 (DHHC5)在少突胶质细胞中下调,减少了TfR1在C98半胱氨酸位点的棕榈酰化。此外,DHHC5在少突胶质细胞中的特异性过表达可显著恢复TfR1的内噬、髓鞘化和铁下垂,从而通过减少铁转运来防止多个脑区域的神经元铁下垂,最终减轻神经损伤。结论我们发现少突胶质细胞DHHC5降低可促进TfR1相关的铁下垂,导致髓鞘退化并引发神经元铁下垂,从而损害新生儿七氟醚暴露后的认知能力。瞬时表达的TfR1可能介导新生儿麻醉易感性的关键时期。这些发现突出了tfr1相关的铁下垂在新生儿麻醉相关神经毒性和少突胶质细胞-神经元相互作用中的关键作用,同时为理解麻醉的暂时性神经毒性提供了新的视角。DHHC5可能是提高新生儿麻醉和铁相关少突胶质细胞疾病安全性的有希望的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
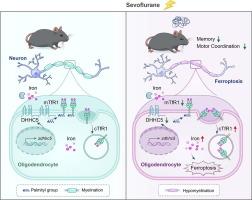
Neonatal sevoflurane exposures inhibits DHHC5-mediated palmitoylation of TfR1 in oligodendrocytes, leading to hypomyelination and neurological impairments
Introduction
Neonatal anesthesia-related neurological impairments are of significant concern, closely linked to oligodendrocyte dysfunction. However, there is a notable temporal discrepancy between the sustained development of oligodendrocytes (myelination) and the short-term vulnerability to anesthesia exposures.Objectives
Given the significant rise in iron demand by oligodendrocytes during neonatal period, our objective was to clarify the potential roles and underlying mechanisms of iron homeostasis, particularly focusing on transferrin receptor 1 (TfR1), in governing the transient susceptibility to anesthesia.Methods
Sevoflurane (3 %, 2 h/day) was administered to wildtype or Pdgfrα-CreERT mice from postnatal day (P)6 to P8. Subsequently, behavioral tests, genetic modulation, co-immunoprecipitation assays, Acyl-resin assisted capture assay and single-cell RNA sequencing were employed on P8 and/or P32.Results
Following neonatal exposure to sevoflurane, the observed cognitive impairments and hypomyelination at P32 were attributed to iron accumulation and ferroptosis, particularly within oligodendrocytes of the corpus callosum (CC). This ferroptosis was mediated by enhanced endocytosis of transiently expressed TfR1, rather than its overexpression, due to inhibited palmitoylation. Among the 21 palmitoyltransferases, only Asp-His-His-Cys5 (DHHC5) was down-regulated in oligodendrocytes, reducing palmitoylation of TfR1 at the C98 cysteine site. Furthermore, specific overexpression of DHHC5 in oligodendrocytes significantly restored TfR1 endocytosis, hypomyelination, and ferroptosis, thereby preventing neuronal ferroptosis across multiple brain regions by decreasing iron transport, ultimately mitigating neurological impairments.Conclusion
We discovered that decreased DHHC5 in oligodendrocytes promotes TfR1 associated ferroptosis, resulting in hypomyelination and initiating neuronal ferroptosis, thereby impairing cognition following neonatal sevoflurane exposures. The transiently expressed TfR1 may mediate the critical period for neonatal anesthesia vulnerability. These findings highlight the pivotal role of TfR1-associated ferroptosis in neonatal anesthesia-associated neurotoxicity and oligodendrocyte-neuron interaction, while providing new perspect to understand temporary neurotoxicity of anesthesia. DHHC5 may represent promising therapeutic target to enhance the safety of neonatal anesthesia and iron-related oligodendrocytes disorders.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


