抗生素对水生微生物组和抗性组的影响和风险阈值的综合评估
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
了解与环境相关的低水平抗生素对水生微生物组和抗性组的影响对人为抗生素污染的风险评估至关重要。在这里,我们研究了7种亚抑制浓度的甲氧苄啶和林可霉素(10 ng/L至10 mg/L)单独和联合对地表水微生物的影响,以未加标的样品为对照。宏基因组测序结果显示,随着抗生素浓度的升高,细菌群落α-多样性降低,而抵抗组α-多样性增加。值得注意的是,细菌群落和抗性体的β-多样性均表现出双相反应,分别在断点浓度为2.73 μg/L和0.68 μg/L时先降低后增加。我们还观察到某些宏基因组组装的抗生素耐药细菌(MAARB)和抗生素耐药基因(ARGs)的浓度依赖性增加,针对OXA-21的甲氧苄啶和针对erm(F)的林可霉素的最小选择浓度(MSCs)分别为2.28 μg/L和32.4 μg/L。在确定引起微生物分类群、抗性组、个体ARGs和MAARB显著变化的风险阈值的各种指标中,抗性组β多样性得出的断点浓度是最保守的。我们建议将这一指标纳入抗生素的环境风险评估框架。我们的研究提供了抗生素对水生微生物组和抗性组的影响的系统评估,为改进水生环境中抗生素污染的风险评估提供了关键见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
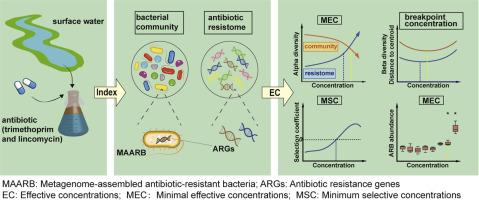
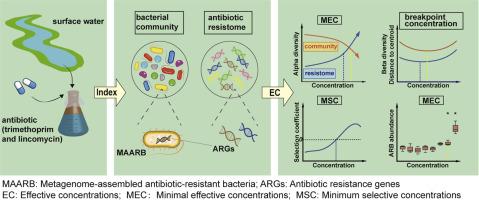
Comprehensive assessment of antibiotic impacts and risk thresholds on aquatic microbiomes and resistomes
Understanding the impacts of environmentally relevant low-level antibiotics on aquatic microbiomes and resistomes is crucial for risk assessment of anthropogenic antibiotic contamination. Here, we investigated the effects of seven subinhibitory concentrations of trimethoprim and lincomycin (10 ng/L to 10 mg/L), individually and in combination, on surface water microcosms over 1 and 7 days, using unspiked samples as controls. Metagenomic sequencing revealed a decrease in bacterial community α-diversity and an increase in resistome α-diversity with rising antibiotic concentrations upon 7 days of exposure. Notably, the β-diversity of both bacterial communities and resistomes exhibited a biphasic response, decreasing and then increasing with breakpoint concentrations of 2.73 µg/L and 0.68 µg/L, respectively. We also observed concentration-dependent increases in certain metagenome-assembled antibiotic-resistant bacteria (MAARB) and antibiotic resistance genes (ARGs), with minimum selective concentrations (MSCs) of 2.28 µg/L for trimethoprim targeting OXA-21 and 32.4 µg/L for lincomycin targeting erm(F). Among various metrics for identifying risk thresholds that induce significant changes in microbial taxa, resistomes, individual ARGs, and MAARB, the breakpoint concentration derived from resistome β-diversity was the most conservative. We propose integrating this metric into environmental risk assessment frameworks for antibiotics. Our study provides a systematic evaluation of antibiotic impacts on aquatic microbiomes and resistomes, offering key insights for refining risk assessments of antibiotic contamination in aquatic environments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


