阴离子取代和界面修饰对氢化物电解质中离子电导率的协同作用
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
全固态电池具有更高的能量密度和安全性,促进了固态电解质的发展。LiBH4具有优异的热力学稳定性,被认为是最有前途的电解质候选者之一。然而,实际应用受到室温下离子电导率低的问题的严重阻碍。为了克服这些问题,我们将Li4(BH4)3I和无定形B2O3结合在一起,得到了一种复合电解质,在30°C时离子电导率达到1.45 × 10-4 S cm-1。B2O3作为晶界填料的加入增强了锂枝晶在固态电解质中的抵抗能力,并在Li4(BH4)3I和B2O3之间产生了高度无序的界面,而没有形成中间相,这是由于Li+转移造成的。该现象增强了氧化稳定性,电位窗口延长为5.0 V。以硫化聚丙烯腈为正极材料组装成锂硫电池,在0.1C条件下循环50次,比放电容量为1308 mA h g-1。该工作将有助于低温固体离子导体的进一步发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
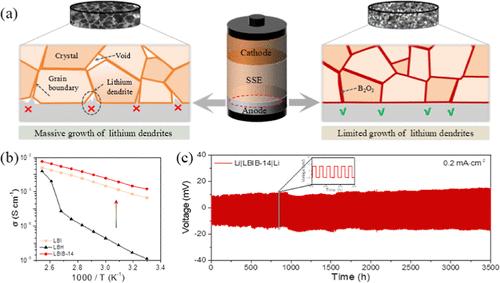
Synergistic Effects of Anion Substitution and Interfacial Modification to Enhance Ionic Conductivity in a Hydride Electrolyte
All-solid-state batteries with a higher energy density and safety promote the development of solid-state electrolytes. LiBH4, which has extraordinary thermodynamic stability with Li, is considered one of the most promising electrolyte candidates. However, practical application is severely hampered by the issue of low ionic conductivity at room temperature. To overcome the problems, Li4(BH4)3I and amorphous B2O3 are combined to attain a composite electrolyte achieving a high ionic conductivity of 1.45 × 10–4 S cm–1 at 30 °C, which greatly inhibits lithium dendrite growth for running the Li symmetric battery over 3500 h. The involvement of B2O3 strengthens the resistance to lithium dendrites in solid-state electrolytes as a grain boundary filler and generates a highly disordered interface between Li4(BH4)3I and B2O3 without the formation of intermediate phase, which is attributed to Li+ transfer. The phenomenon strengthens oxidation stability with an extending potential window of 5.0 V. A lithium–sulfur battery was assembled with sulfurized polyacrylonitrile as the cathode material, which exhibits a specific discharge capacity of 1308 mA h g–1 after 50 cycles at 0.1C. The work will contribute to the further development of low-temperature solid-state ionic conductors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


