巨噬细胞和T细胞协同抗肿瘤的双重刺激反应纳米免疫调节剂
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
只有少数患者受益于目前以t细胞为重点的适应性免疫疗法,这强调了利用先天免疫细胞,特别是巨噬细胞,进行多层肿瘤控制的必要性。然而,能够协调多个免疫细胞的高效疗法仍然很少。本文提出了一种双刺激反应纳米免疫调节剂(6EPP@si),专门针对肿瘤微环境(TME),用于巨噬细胞和T细胞的抗肿瘤协同作用。利用功能性聚合物载体,我们将内质网定位的光敏剂6E和靶向CD47的小干扰RNA (siCD47)共同递送到乳腺肿瘤中。在酸性和高谷胱甘肽TME中,6EPP@si经历自我溶酶体逃逸和纳米裂解,以实现精确的按需药物释放。因此,释放到细胞质中的siCD47使CD47有效沉默,而内质网靶向光敏剂6E通过基于活性氧的内质网应激诱导免疫原性细胞死亡,触发损伤相关分子模式的释放,包括钙网蛋白表面易位。6EPP@si通过调节抗吞噬和前吞噬信号增强巨噬细胞吞噬,并促进抗原呈递激活T细胞。在原位乳腺肿瘤和自发性肺转移瘤模型中,该联合方法显示出强大的抗肿瘤作用和有效的抗转移免疫,为同时激活多个免疫细胞以增强癌症免疫治疗提供了有意义的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
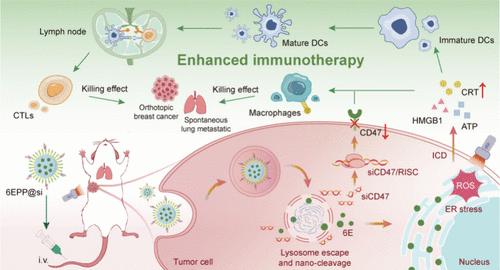
A Dual Stimuli-Responsive Nanoimmunomodulator for Antitumor Synergy of Macrophages and T Cells
Only a minority of patients benefit from current T-cell-focused adaptive immunotherapies, underscoring the need to engage innate immune cells, particularly macrophages, for multilayered tumor control. However, high-efficacy therapeutics capable of orchestrating multiple immune cells remain scarce. Herein, a dual stimuli-responsive nanoimmunomodulator (6EPP@si) that caters specifically to the tumor microenvironment (TME) is presented for the antitumor synergy of macrophages and T cells. Using the functional polymer-based carrier, we co-deliver the endoplasmic reticulum (ER)-localized photosensitizer 6E and small interfering RNA targeting CD47 (siCD47) into breast tumors. Within the acidic and high-glutathione TME, 6EPP@si undergoes self-lysosome escape and nanocleavage for precise, on-demand drug release. Consequently, siCD47 released into the cytoplasm enables potent CD47 silencing, while the ER-targeted photosensitizer 6E induces immunogenic cell death through reactive oxygen species-based ER stress, triggering the release of damage-associated molecular patterns, including calreticulin surface translocation. 6EPP@si enhances macrophage phagocytosis by modulating both antiphagocytic and prophagocytic signals and also promotes antigen presentation to activate T cells. In orthotopic breast tumor and spontaneous lung metastatic tumor models, this combined approach demonstrates robust antitumor effects and effective antimetastatic immunity, offering a meaningful strategy to simultaneously activate multiple immune cells for enhancing cancer immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


