Pd或双催化直接n-烯基化反应条件控制发散选择性合成(Z)- n-乙烯基和n-烯基苯并咪唑
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们开发了一种条件控制的由1,1,3-三苯基丙-2-炔-1-醇和苯并咪唑通过Pd或双催化的n-烯基化反应合成(Z)- n-乙烯基和n-烯基苯并咪唑的方法,包括亲核攻击和C-C键裂解过程。该方法具有立体定向合成、官能团耐受性好、底物范围广、效率高等特点,可方便、选择性地合成所需的两种不同产物。该策略为合成具有生物活性和药物活性的咪唑杂环化合物提供了显著的优势。本文章由计算机程序翻译,如有差异,请以英文原文为准。
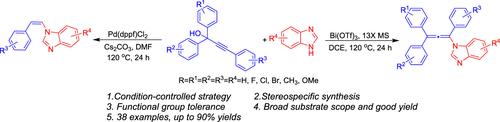
Condition-Controlled Divergent Selective Synthesis of (Z)-N-Vinyl and N-Allenyl Benzimidazoles by Pd- or Bi-Catalyzed Direct N-Alkenylation Reactions
We have developed a condition-controlled divergent synthesis of (Z)-N-vinyl and N-allenyl benzimidazoles from 1,1,3-triphenylprop-2-yn-1-ols and benzimidazoles through Pd- or Bi-catalyzed N-alkenylation reactions involving nucleophilic attack and C–C bond cleavage processes. The desired two different kinds of products can be conveniently and selectively synthesized by using this strategy, which features stereospecific synthesis, good functional group tolerance, a broad substrate scope, and high efficiency. The strategy provides significant advantages for the synthesis of biologically and pharmaceutically active imidazoheterocycles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


