通过亲电性反转铁催化烯基锌化
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
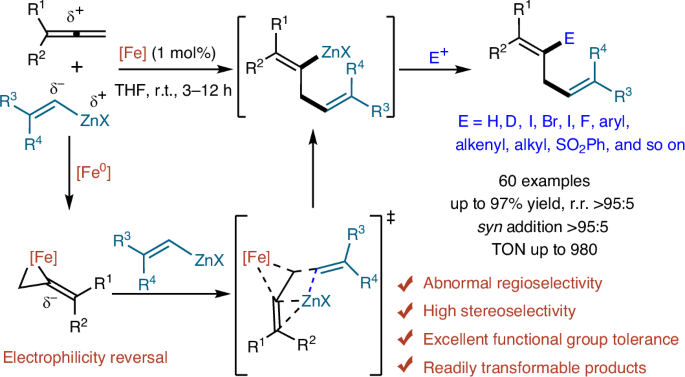
由于烯的结构特点,亲核加成通常发生在烯缺乏电子的中心碳原子上。在这里,我们报道了一个铁催化的末端烯基锌化反应,该反应显示出异常的区域选择性,其中来自有机锌试剂的亲电性锌部分被结合在烯的缺电子中心碳原子上。该烯基锌化反应具有广泛的官能团相容性和良好的区域选择性和立体选择性。利用这种方法,我们获得了顺式-1,4-二烯锌试剂及其相应的多取代的1,4-二烯衍生物,这是众所周知的难以通过常规路线制备的。机理研究表明,Fe(0)通过π背键给电子,使得烯烯碳的亲电性发生了意想不到的逆转,从而导致了所观察到的异常区域选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Iron-catalysed alkenylzincation of allenes via electrophilicity reversal
Given the structural characteristics of allenes, nucleophilic additions usually occur at the electron-deficient central carbon atom of allene. Here we report an iron-catalysed alkenylzincation reaction of terminal allenes that shows abnormal regioselectivity, wherein the electrophilic zinc moiety from an organozinc reagent is incorporated at the electron-deficient central carbon atom of the allene. This alkenylzincation reaction shows broad functional group compatibility and excellent regio- and stereoselectivities. Using this method, we accessed cis-1,4-dienylzinc reagents and their corresponding polysubstituted 1,4-diene derivatives, which are notoriously challenging to prepare via conventional routes. Mechanistic studies revealed that an unexpected reversal of the electrophilicity of the allene carbons is realized through the electron donation from the Fe(0) to the allene via π back-bonding, resulting in the observed abnormal regioselectivity. Allenes are versatile substrates in synthetic chemistry. Now an iron catalyst enables unusual regioselectivity by mediating an electrophilicity reversal of allenes for stereoselective alkenylzincation reactions affording 1,4-diene zinc products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


