非晶和结晶电催化剂水氧化差异的来源
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
非晶催化剂在析氧反应(OER)中的表现与晶体催化剂不同;然而,这种差异的起源仍然是模糊的。本文以非晶型和结晶型CoOOH为模型催化剂,探讨其OER性能差异的原因。电化学测量结果表明,在OER初始阶段,无定形CoOOH比结晶CoOOH具有更多的活性位点,但每个位点的本征活性较低。然而,当OER继续进行时,非晶CoOOH的每个位点的本征活性继续增加,直到达到接近结晶CoOOH的水平。在对其进行表征和电化学分析的基础上,提出了一种双途径重构模型来解释这些CoOOH的催化行为。催化剂的本征活性主要由两种重构途径控制。无定形和结晶CoOOH的内在活性的区别是由OER中包含的每个途径的不同比例引起的。此外,Co4+与非晶催化剂中的氧空位之间的猝灭反应激发了表面重构,从而提高了结晶度。本研究为理解OER的表面重建机制提供了新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
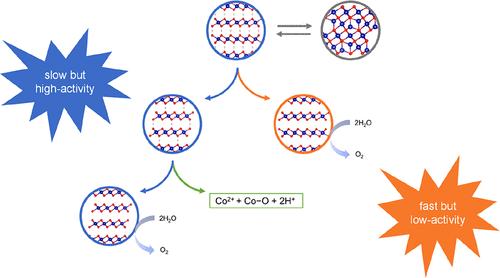
Origin of Disparities in Water Oxidation between Amorphous and Crystalline Electrocatalysts
Amorphous catalysts behave differently in oxygen evolution reaction (OER) performance compared with their crystalline counterparts; however, the origin of this disparity is still ambiguous. Herein, amorphous and crystalline CoOOH are invoked as the model catalysts to explore the origin of their difference in the OER performance. Electrochemical measurement results demonstrate that the amorphous CoOOH has more active sites in quantity but lower intrinsic activity per site than the crystalline CoOOH in the initial stage of the OER. Nevertheless, the intrinsic activity per site of the amorphous CoOOH continues to increase until a level close to that of the crystalline CoOOH is achieved when the OER proceeds. On the basis of operando characterizations and electrochemical analysis, a dual-pathway model of reconstruction is proposed to explain the catalytic behaviors of these CoOOH. The intrinsic activity of catalysts is dominated by two reconstruction pathways. The distinction of intrinsic activity between the amorphous and crystalline CoOOH is caused by the different proportions of each pathway included in OER. Moreover, the quenching reaction between Co4+ and the oxygen vacancy in the amorphous catalyst motivates the surface reconstruction and subsequently promotes the crystallinity. This study provides a perspective for understanding the surface reconstruction mechanism in the OER.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


