CYP2C19对奥美拉唑对映体的立体和区域选择性代谢:来自Curtin-Hammett框架下结合自由能和QM/MM计算的见解
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
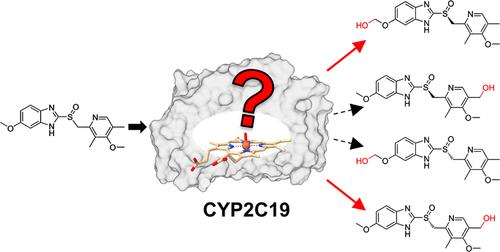
奥美拉唑是一种广泛用于治疗酸相关疾病的药物,它是R-和s -对映体的外消旋混合物。奥美拉唑的治疗效果受这些对映体代谢稳定性差异的影响。两种人类细胞色素P450亚型在其代谢中起关键作用:CYP2C19优先羟化r -对映体生成5-羟基奥美拉唑,而CYP3A4主要代谢s -对映体(埃索美拉唑)生成奥美拉唑砜。此外,CYP2C19在5 ' -甲氧基位置代谢埃索美拉唑,生成5 ' - o -羟基奥美拉唑,随后水解为5 ' - o -去甲基奥美拉唑。尽管这些代谢途径具有临床意义,但代谢选择性的机制和决定因素仍然知之甚少。在本研究中,我们引入了一种基于热力学积分的结合自由能计算和混合量子力学/分子力学(QM/MM)计算相结合的计算方案,研究了CYP2C19对两种奥美拉唑对映体的立体和区域选择性代谢。这种集成的协议在模拟中结合了Curtin-Hammett原理,使代谢选择性的高效和准确的建模成为可能。发现水介导的氢键网络在决定底物结合亲和力方面起着重要作用。此外,四种代谢途径的活化能障碍不同,突出了活性位点环境在调节底物羟基化反应性中的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Stereo- and Regioselective Metabolism of the Omeprazole Enantiomers by CYP2C19: Insights from Binding Free Energy and QM/MM Calculations in a Curtin–Hammett Framework
Omeprazole, a widely used drug for acid-related disorders, is administered as a racemic mixture of its R- and S-enantiomers. The therapeutic efficacy of omeprazole is influenced by the differential metabolic stability of these enantiomers. Two human cytochrome P450 isoforms play critical roles in its metabolism: CYP2C19 preferentially hydroxylates the R-enantiomer to produce 5-hydroxyomeprazole, while CYP3A4 predominantly metabolizes the S-enantiomer (esomeprazole) to generate omeprazole sulfone. Additionally, CYP2C19 metabolizes esomeprazole at the 5′-methoxy position, yielding 5′-O-hydroxyomeprazole, which subsequently hydrolyzes to 5′-O-desmethylomeprazole. Despite the clinical significance of these metabolic pathways, the mechanisms and determinants underlying metabolic selectivity remain poorly understood. In this study, we introduced a computational protocol that integrates binding free energy calculations based on thermodynamic integration and hybrid quantum mechanics/molecular mechanics (QM/MM) calculations to investigate the stereo- and regioselective metabolism of the two omeprazole enantiomers by CYP2C19. This integrated protocol incorporates the Curtin–Hammett principle in simulations, enabling efficient and accurate modeling of metabolic selectivity. Water-mediated H-bond networks were found to play a significant role in determining substrate binding affinity. Furthermore, the activation energy barriers for the four metabolic pathways differ, highlighting the pivotal role of the active-site environment in modulating substrate hydroxylation reactivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


