揭示电催化NO3RR中钴硫化物的结构演变:Cl -的不可避免的影响
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
电化学硝酸还原(NO3RR)制氨是缓解NO3 -污染和生产有价值NH3的一种有吸引力的方法。钴硫化合物被广泛认为是NO3RR的潜在电催化剂。然而,对于钴基硫化物在催化过程中可能的结构演变、长期稳定性和反应位点的研究仍然缺乏。本文采用三种不同硫含量的硫化钴(CoSx,其中x = 8/ 9,2,1.097)作为碱性条件下电催化NO3RR的催化剂。在−0.8 V vs RHE条件下,这些CoSx均表现出良好的性能,法拉第效率达到80%,NH3产率达到1780 mmol h-1 gcat-1。通过x射线衍射(XRD)、透射电镜(TEM)等表征,发现这些硫化钴在NO3RR过程中很容易转化为氢氧化钴。这一现象似乎与热力学预测相矛盾,根据Pourbaix图,这些CoSx化合物即使在催化条件下也应该是稳定的。我们认为这是由于电解质中Cl -离子的存在促进了CoSx向Co(OH)2的转化。氯离子在工业环境和自然水体中都很常见,很难去除。进化的Co(OH)2物种被认为是催化NO3RR的主要物质,特别是在长期催化过程中。本研究突出了当前碱性电催化NO3RR条件下CoSx催化剂不可避免的结构演变,为未来催化剂的明智选择和设计提供理论指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
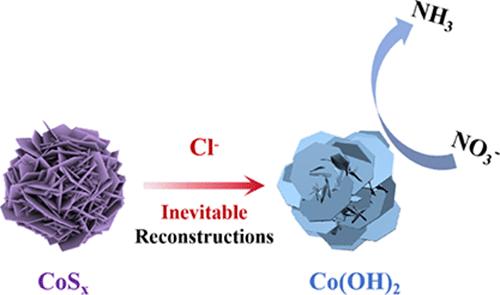
Unraveling the Structural Evolution of Cobalt Sulfides in Electrocatalytic NO3RR: the Inescapable Influence of Cl–
Electrochemical nitrate reduction (NO3RR) to ammonia is an attractive approach for mitigating NO3– pollution and producing valuable NH3. Cobalt–sulfur compounds are widely considered to be potential electrocatalysts for NO3RR. However, there is still a lack of research on the probable structural evolution, long-term stability, and reactive sites of cobalt-based sulfides during catalysis. Herein, we have employed three cobalt sulfides (CoSx, where x = 8/9, 2, 1.097) with different sulfur contents as catalysts for electrocatalytic NO3RR under alkaline conditions. At −0.8 V vs RHE, all these CoSx show promising performances that Faradaic efficiencies of >80% and a high yield of >1780 mmol h–1 gcat–1 for NH3 production are achieved. Through a combination of X-ray diffraction (XRD), transmission electron microscopy (TEM), and other characterizations, it is revealed that all these cobalt sulfides are easily converted into cobalt hydroxide during the NO3RR. This phenomenon is seemingly contradictory to the thermodynamic prediction that, according to the Pourbaix diagram, these CoSx compounds should be stable even under the catalytic condition. We suggest that this is due to the presence of Cl– ions in the electrolyte that promote the transformation of CoSx toward Co(OH)2. Chloride ions are commonly found in both industrial settings and natural water bodies and are challenging to remove. The evolved Co(OH)2 species is proposed to be responsible for catalyzing NO3RR, especially during a long-term catalytic process. This study highlights the inevitable structural evolution of CoSx catalysts under current alkaline electrocatalytic NO3RR conditions, offering theoretical guidance for the judicious selection and design of future catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


