比较基因组学提供了对马蹄铁蝙蝠的染色体进化和免疫适应的见解
IF 13.9
1区 生物学
Q1 ECOLOGY
引用次数: 0
摘要
马蹄蝠是人畜共患病毒的天然宿主,但其抗病毒免疫的遗传基础尚不清楚。在这里,我们生成了两个新的染色体水平的马蹄铁蝙蝠物种(Rhinolophus)和三个近亲的基因组组装,并表明,在其多样化过程中,马蹄铁蝙蝠经历了广泛的染色体重排和与片段复制相关的基因扩增。这些扩增在I型干扰素和干扰素刺激基因ANXA2R中产生了新的适应性变异,正如我们的功能测定所表明的那样,这些变异可能增强抗病毒状态。全基因组选择筛选,包括候选渗入区域,揭示了许多假定的与免疫相关的分子适应,包括病毒受体。通过将分类单元覆盖范围扩大到10个马蹄蝠物种,我们确定了SARS-CoV-2受体ACE2的新变体,并报告了收敛的功能重要残基,这些残基可以解释哺乳动物之间更广泛的易感性模式。我们得出的结论是,马蹄蝙蝠在具有多种和多尺度突变变化的基因组区域中具有许多适应特征,包括一些可能与病毒免疫反应相关的特征。本文章由计算机程序翻译,如有差异,请以英文原文为准。

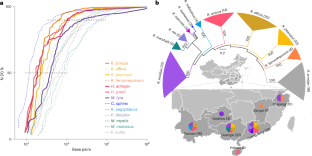
Comparative genomics provides insights into chromosomal evolution and immunological adaptation in horseshoe bats
Horseshoe bats are natural hosts of zoonotic viruses, yet the genetic basis of their antiviral immunity is poorly understood. Here we generated two new chromosomal-level genome assemblies for horseshoe bat species (Rhinolophus) and three close relatives, and show that, during their diversification, horseshoe bats underwent extensive chromosomal rearrangements and gene expansions linked to segmental duplications. These expansions have generated new adaptive variations in type I interferons and the interferon-stimulated gene ANXA2R, which potentially enhance antiviral states, as suggested by our functional assays. Genome-wide selection screens, including of candidate introgressed regions, uncover numerous putative molecular adaptations linked to immunity, including in viral receptors. By expanding taxon coverage to ten horseshoe bat species, we identify new variants of the SARS-CoV-2 receptor ACE2, and report convergent functionally important residues that could explain wider patterns of susceptibility across mammals. We conclude that horseshoe bats have numerous signatures of adaptation, including some potentially related to immune response to viruses, in genomic regions with diverse and multiscale mutational changes. A comparative analysis of bat genomes, including five newly assembled chromosome-scale genomes, provides insights into the diversification of horseshoe bats and reveals molecular adaptations with a potential role in antiviral immune response.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature ecology & evolution
Agricultural and Biological Sciences-Ecology, Evolution, Behavior and Systematics
CiteScore
22.20
自引率
2.40%
发文量
282
期刊介绍:
Nature Ecology & Evolution is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences. Nature Ecology & Evolution provides a place where all researchers and policymakers interested in all aspects of life's diversity can come together to learn about the most accomplished and significant advances in the field and to discuss topical issues. An online-only monthly journal, our broad scope ensures that the research published reaches the widest possible audience of scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


