IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
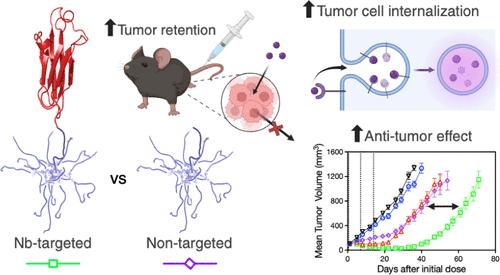
树枝状聚合物是一种支化的大分子结构,是癌症治疗等小分子药物的有用纳米载体。树枝状聚合物体积小,可渗透到实体瘤中,加上功能化的低污点 PEG 涂层,可最大限度地减少瞬时细胞相互作用,并延长血浆循环时间。虽然 PEG 化树枝状分子作为抗癌疗法前景广阔,但通过在树枝状分子上共轭细胞靶向配体,还有可能提高肿瘤细胞的特异性并促进细胞对药物的吸收。为了实现这一目标,我们利用扩展的遗传密码和生物正交点击化学将单甲基金丝桃素 E(MMAE)负载的 PEG 化树枝状分子功能化,每个树枝状分子含有一个肿瘤细胞靶向纳米抗体。单一纳米抗体配体的均匀添加有利于HER2阳性靶细胞对药物载荷的更大细胞内吸收,同时保留了树枝状聚合物理想的循环特性。与未修饰的树枝状聚合物相比,纳米抗体-树枝状聚合物共轭物在24小时内的肿瘤浸润水平相似,但靶向树枝状聚合物对肿瘤生长的抑制作用明显更强,在肿瘤内的长期存留也更持久。我们的研究结果表明,仅进行生物分布研究并不能很好地预测治疗效果。本文介绍的受控共轭策略保留了树枝状聚合物的尺寸优势和组织穿透性,同时在难以进入的实体瘤组织中最大限度地提高了靶向细胞摄取和药效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Nanobody-Mediated Cellular Uptake Maximizes the Potency of Polylysine Dendrimers While Preserving Solid Tumor Penetration
Dendrimers are branched macromolecular structures that are useful nanocarriers for small-molecule drugs, such as cancer therapeutics. Their small size permits penetration into solid tumors, coupled with functionalization with a low-fouling PEG coating that minimizes transient cellular interactions and enhances plasma circulation time. While PEGylated dendrimers show significant promise as anticancer therapeutics, there is potential to increase tumor cell specificity and drive uptake of drugs into cells by conjugating cell-targeting ligands onto the dendrimers. To achieve this, we used an expanded genetic code and bio-orthogonal click chemistry to functionalize monomethyl auristatin E (MMAE)-loaded PEGylated dendrimers with a single tumor cell-targeting nanobody per dendrimer. The uniform addition of a single nanobody ligand facilitated greater intracellular uptake of the drug payload into HER2-positive target cells, while preserving the desirable circulatory characteristics of dendrimers. While the nanobody–dendrimer conjugates show similar levels of tumor infiltration over 24 h compared to unmodified dendrimers, the targeted dendrimers had significantly greater inhibition of tumor growth and long-term retention in the tumors. Our results highlight that biodistribution studies alone are poor predictors of therapeutic performance. The controlled conjugation strategy presented here preserves the size advantage and tissue penetration of dendrimers while maximizing targeted cellular uptake and potency in difficult-to-access solid tumor tissue.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


