在稳态和再生条件下,神经干细胞的静止和激活动力学是由其后代的反馈输入调节的
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
干细胞的终身维持意味着来自生态位的反馈机制调节了它们的静止/激活动力学。在小鼠成年脑室下神经干细胞(NSC)生态位中,我们绘制了多个生态位细胞诱导的NSCs功能反应的精确时空图,并使用机器学习来预测NSC与特定生态位细胞类型的相互作用。我们揭示了一种反馈机制,即瞬时扩增细胞(瞬时扩增细胞)及其快速分裂的后代直接抑制NSC增殖状态。NSC过程包裹在tap周围,并在其接触点显示Ca2+活性热点,由ephrin (Efn)信号介导。Efn信号的调节或TAP的消融改变了NSCs的Ca2+信号,导致它们的激活。体内光遗传调节Ca2+动力学废除NSC激活和阻止生态位补充。因此,tap - nsc反馈信号控制着干细胞的静止和激活,提供了一种在生命中维持干细胞池的机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
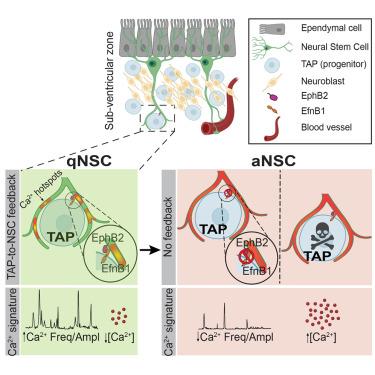
Neural stem cell quiescence and activation dynamics are regulated by feedback input from their progeny under homeostatic and regenerative conditions
Life-long maintenance of stem cells implies that feedback mechanisms from the niche regulate their quiescence/activation dynamics. Here, in the mouse adult subventricular neural stem cell (NSC) niche, we charted a precise spatiotemporal map of functional responses in NSCs induced by multiple niche cells and used machine learning to predict NSC interactions with specific niche cell types. We revealed a feedback mechanism whereby the NSC proliferative state is directly repressed by transient amplifying cells (TAPs), their rapidly dividing progeny. NSC processes wrap around TAPs and display hotspots of Ca2+ activity at their points of contact, mediated by ephrin (Efn) signaling. The modulation of Efn signaling or TAP ablation altered the Ca2+ signature of NSCs, leading to their activation. In vivo optogenetic modulation of Ca2+ dynamics abrogated NSC activation and prevented niche replenishment. Thus, TAP-to-NSC feedback signaling controls stem cell quiescence and activation, providing a mechanism to maintain stem cell pools throughout life.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


