机械敏感通道YbiO的电导相当于最大的门控孔
IF 4.3
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
细菌机械敏感通道分为大电导(MscL)和小电导(类mscs)家族。间充质干细胞和间充质干细胞的功能是通过充当压力安全阀来保护细胞免受渗透休克。在类mscs家族中,大肠杆菌编码更大的通道,如YbiO、MscK和MscM,但它们的生理作用尚不清楚。与msl相比,它们的电导据报道低3-10倍。我们发现YbiO可以实现~ 3ns的电导,等效孔径为>;直径为25 Å,等于已知的最大门控孔MscL。我们在低温电子显微镜(cryo-EM)下确定了YbiO的亚开放构象结构,证明了多个亚态的存在。一个亚态与我们的结构的孔隙开放程度一致,而另一个则与之前认为的完全开放的状态相匹配。我们的发现展示了令人惊讶的能力,暗示了YbiO和其他潜在的msc样通道的新生理作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
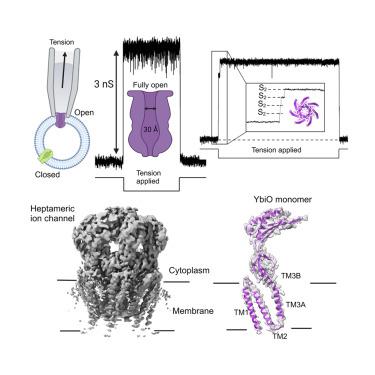
The mechanosensitive channel YbiO has a conductance equivalent to the largest gated-pore
Bacterial mechanosensitive channels are divided into large (MscL) and small (MscS-like) conductance families. The function of MscS and MscL is to protect cells against osmotic shock by acting as pressure safety valves. Within the MscS-like family, E. coli encodes much larger channels, such as YbiO, MscK, and MscM, but their physiological role remains unclear. Compared to MscL their conductances are reported as 3–10 times lower. We show that YbiO can achieve a conductance of ∼3 nS, and an equivalent pore opening of > 25 Å in diameter, equaling the known largest gated pore, MscL. We determine a cryoelectron microscopy (cryo-EM) structure of YbiO in a sub-open conformation, demonstrating the existence of multiple substates. One substate is consistent with the pore opening extent of our structure and the other matches states previously thought to resemble full openings. Our findings demonstrate surprising capabilities, hinting at new physiological roles for YbiO and potentially other MscS-like channels.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


