水的同位素标记揭示了电化学CO2还原过程中氢的转移途径
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
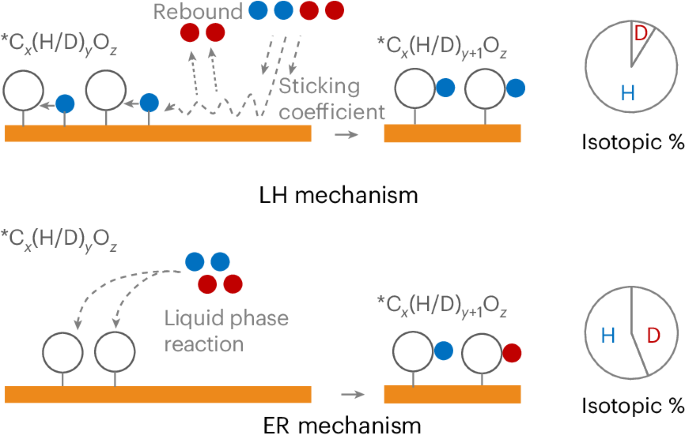
了解电化学CO2还原过程中的加氢途径对控制产物选择性具有重要意义。质子耦合电子从溶剂水直接转移的elei - rideal机制通常被认为是氢转移的主要途径。然而,原则上,氢化也可以通过Langmuir-Hinshelwood机制发生,利用表面吸附的*H。在这里,通过在H2O-D2O混合物中使用Cu进行CO2还原,我们提出了Langmuir-Hinshelwood机制可能是主要加氢途径的证据。由此,我们估计了每种机制对六种重要二氧化碳还原产物形成的贡献程度。通过计算模拟,我们发现C-H键和O-H键的形成分别受Langmuir-Hinshelwood和Eley-Rideal机制的支配。我们还表明,促进Eley-Rideal途径可能对选择性多碳产物形成和抑制氢演化至关重要。这些发现为二氧化碳还原途径的理论建模和电催化剂设计引入了重要的考虑因素。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Isotopic labelling of water reveals the hydrogen transfer route in electrochemical CO2 reduction
Understanding the hydrogenation pathway in electrochemical CO2 reduction is important for controlling product selectivity. The Eley–Rideal mechanism involving proton-coupled electron transfer directly from solvent water is often considered to be the primary hydrogen transfer route. However, in principle, hydrogenation can also occur via the Langmuir–Hinshelwood mechanism using surface-adsorbed *H. Here, by performing CO2 reduction with Cu in H2O–D2O mixtures, we present evidence that the Langmuir–Hinshelwood mechanism is probably the dominant hydrogenation route. From this, we estimate the extent to which each mechanism contributes towards the formation of six important CO2 reduction products. Through computational simulations, we find that the formation of C–H bonds and O–H bonds is governed by the Langmuir–Hinshelwood and Eley–Rideal mechanism, respectively. We also show that promoting the Eley–Rideal pathway could be crucial towards selective multicarbon product formation and suppressing hydrogen evolution. These findings introduce important considerations for the theoretical modelling of CO2 reduction pathways and electrocatalyst design. CO2 electroreduction to higher-value carbons can occur through adsorbed hydrogen or through proton-coupled electron transfer from water. Understanding the impact of each route on product selectivity is challenging. Now H/D isotopic labelling reveals the contribution of each mechanism towards product formation and shows that adsorbed hydrogen dominates the reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


