天然Diels-Alderase的氨催化作用
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
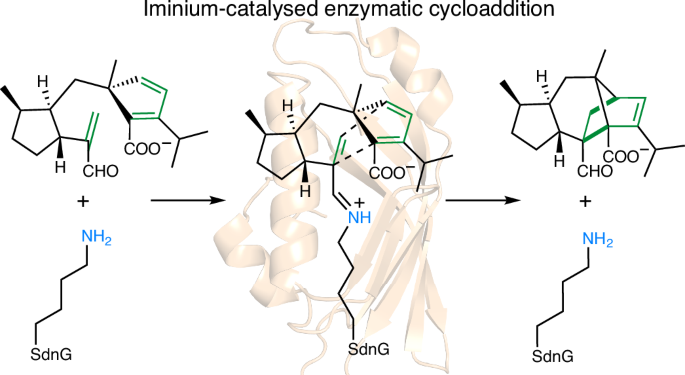
微量催化环加成是有机催化中最突出的例子之一,然而,尽管在酶中广泛存在着微量加合物,但生物上的对应物尚未被报道。本文提出了天然Diels-Alderase SdnG催化降冰片烯形成的生化、结构和计算证据。通过结构分析发现,在活性位点K127的ε-氮与烯醛亲二酚的醛基之间存在席夫碱加合物,并在催化条件下通过硼氢化物还原捕获。这种希夫碱加合物使底物处于近攻击构象,并通过亲二酚活化使diols - alder环化的过渡态势垒降低8.3 kcal mol−1。提出了一个由催化三元组组成的氢键网络,以促进形成铝所需的质子转移。这项工作为diels - alderase建立了一种有趣的催化模式,并为设计低基(生物)催化剂指明了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Iminium catalysis in natural Diels–Alderase
Iminium-catalysed cycloaddition is one of the most prominent examples of organocatalysis, yet a biological counterpart has not been reported, despite the widespread occurrence of iminium adducts in enzymes. Here we present biochemical, structural and computational evidence for iminium catalysis by the natural Diels–Alderase SdnG, which catalyses norbornene formation in sordarin biosynthesis. A Schiff-base adduct between the ε-nitrogen of active site K127 and the aldehyde group of the enal dienophile is revealed by structural analysis and captured under catalytic conditions via borohydride reduction. This Schiff-base adduct positions the substrate into near-attack conformation and decreases the transition-state barrier of Diels–Alder cyclization by 8.3 kcal mol−1 via dienophile activation. A hydrogen-bond network consisting of a catalytic triad is proposed to facilitate the proton transfer required for iminium formation. This work establishes an intriguing mode of catalysis for Diels–Alderases and points the way to the design of iminium-based (bio)catalysts. Iminium-catalysed cycloaddition is a prominent example of organocatalytic reactivity, yet a biological counterpart has not been identified. Now, the authors report biochemical, structural and computational evidence for iminium catalysis by the natural Diels–Alderase SdnG.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


