多种变构机制抑制PRC2活性基因的活性
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
prc2依赖性H3K27甲基化必须从活性基因中排除,以支持适当的转录程序。PRC2与核小体底物结合的结构包含与活性转录相关的组蛋白修饰,解释了PRC2如何在活性基因中被抑制。本文章由计算机程序翻译,如有差异,请以英文原文为准。

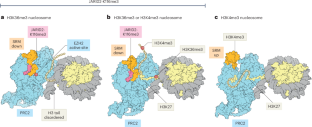
Multiple allosteric mechanisms suppress PRC2 activity at active genes
PRC2-dependent H3K27 methylation must be excluded from active genes to support appropriate transcriptional programs. Structures of PRC2 bound to nucleosome substrates containing histone modifications associated with active transcription explain how PRC2 is inhibited at active genes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


