沙门氏菌压力和持久性对抗生素清除的影响有限
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
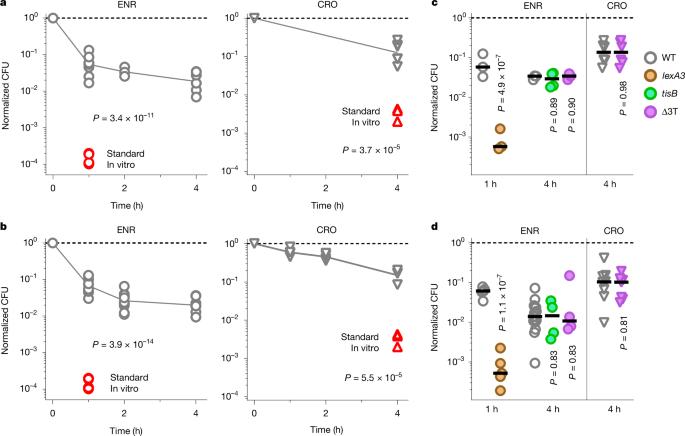
抗菌化合物对控制细菌感染至关重要。在实验室条件下,应激诱导的细菌耐受性和持久性可以破坏抗菌活性,但其在生理条件下的定量效应尚不清楚1,2。在这里,我们确定了受感染小鼠的抗菌剂和组织模拟趋化剂对沙门氏菌清除的限制。抗生素恩诺沙星和头孢曲松在两种条件下均表现出较差的抗沙门氏菌活性,这主要是由于严重的营养饥饿,限制了沙门氏菌的繁殖3,4,5。其他与感染相关的条件,如酸性pH值、葡萄糖、氧化应激、亚硝化应激、抗菌肽、渗透压、限氧、二氧化碳和碳酸盐,以及药物外排、毒素-抗毒素模块和细胞大小的影响有限。基于沙门氏菌菌落形成单位的双相下降,弹性沙门氏菌的一个子集似乎是恩诺沙星清除的关键障碍。然而,这些数据具有误导性,因为蜂群的形成与大量暴露后的杀戮相混淆。更精确的单细胞实时分析显示,损伤一致缓慢,表明整个沙门氏菌种群具有很高的恢复能力。由此产生的大量细菌的生存将超弹性持久性细菌的影响降至最低。因此,饥饿引起的沙门氏菌的一般恢复力是抗生素清除不良的主要原因。这些发现强调了在生理条件下用实时单细胞测定定量抗生素活性的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Limited impact of Salmonella stress and persisters on antibiotic clearance
Antimicrobial compounds are essential for controlling bacterial infections. Stress-induced bacterial tolerance and persisters can undermine antimicrobial activities under laboratory conditions, but their quantitative effects under physiological conditions remain unclear1,2. Here we determined constraints on clearance of Salmonella by antimicrobials in infected mice and tissue-mimicking chemostats. The antibiotics enrofloxacin and ceftriaxone exhibited poor anti-Salmonella activity under both conditions, primarily owing to severe nutrient starvation, which restricted Salmonella replication3–5. Other infection-associated conditions, such as acidic pH, glucose, oxidative stress, nitrosative stress, antimicrobial peptides, osmolarity, oxygen limitation, carbon dioxide and carbonate, as well as drug efflux, toxin–antitoxin modules and cell size had limited effects. A subset of resilient Salmonella appeared as a key obstacle for clearance by enrofloxacin, based on the biphasic decline of Salmonella colony-forming units. However, these data were misleading, because colony formation was confounded by extensive post-exposure killing. More accurate single-cell, real-time assays showed uniformly slow damage, indicating high resilience across the entire Salmonella population. The resulting extensive survival of bulk bacteria minimized the effect of hyper-resilient persisters. Thus, starvation-induced general resilience of Salmonella was the main cause of poor antibiotic clearance. These findings highlight the importance of quantifying antibiotic activity with real-time, single-cell assays under physiological conditions. Experiments in infected mice and tissue-mimicking chemostats show that resilience of Salmonella against antimicrobial compounds is largely a result of starvation-induced general resilience rather than survival of a small subset of hyper-resilient cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


