通过闭环回收提高 PET 糖酵解的可持续性
IF 4.1
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
对于聚对苯二甲酸乙二醇酯(PET)的化学回收而言,乙二醇分解是一种温和的工艺,但由于废液量大,其效率仍然不高。因此,这项工作的目的是促进乙二醇(EG)反应物/溶剂、均匀溶解的醋酸锌(ZnAc2)催化剂以及溶解产物对苯二甲酸二(2-羟乙基)酯(BHET)在无水 PET 糖解过程中的循环利用。通过再计量和洗涤相结合的方法,我们节省了 48.6 ± 0.5% 的 EG 和 50.0 ± 5.2% 的 ZnAc2。通过在 BHET 干燥/再聚合过程中收集蒸发的 EG,在五次重复运行中,每次还可额外节省 35% 的 EG。在循环实验中采用优化的洗涤程序,150 分钟的反应时间后,PET 转化率始终保持在平衡状态。此外,在回收运行中,整体工艺产量保持在 80.6 ± 1.8%,废物流的产生量也降到了最低。这项研究凸显了内部循环利用的可行性和环保优势,为实现更具可持续性和成本效益的 PET 糖酵解工艺铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。

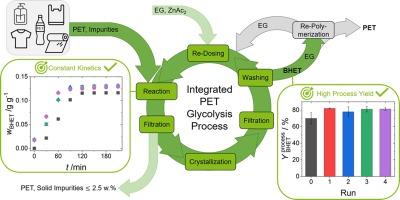
Enhancing sustainability in PET glycolysis by closed-loop recycling
Glycolysis is a mild process for the chemical recycling of poly(ethylene terephthalate) (PET), but still lacks efficiency due to high waste streams. The aim of this work therefore is to facilitate recycling of ethylene glycol (EG) reactant/solvent and homogeneously dissolved zinc acetate (ZnAc2) catalyst as well as dissolved product bis(2-hydroxyethyl)terephthalate (BHET) in the water-free PET glycolysis process. Through a combined approach of re-dosing and washing we saved 48.6 ± 0.5% of EG and 50.0 ± 5.2% of ZnAc2. By incorporating the collection of evaporated EG during BHET drying/re-polymerization, additional 35% of EG could further be saved in each of the five duplicate runs. While applying an optimized washing procedure in the recycling experiments, an equilibrium PET conversion was consistently reached after 150 min of reaction time. Furthermore, the overall process yield was maintained at 80.6 ± 1.8% in the recycling runs and the waste stream generation was reduced to a minimum. This study highlights the viability and environmental advantages of internal recycling, paving the way for a more sustainable and cost-effective PET glycolysis process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


