具有细胞穿透和抗菌双重功能的自组装肽,是对抗细胞内细菌的纳米武器
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
Science Advances
Pub Date : 2025-02-05
引用次数: 0
摘要
细胞内细菌感染和抗菌素耐药性正威胁着全球公共卫生系统。抗菌肽是对抗细菌耐药性的潜在解决方案,但设计具有细胞穿透和抗菌特性双重功能的自组装纳米肽来对抗细胞内细菌仍然是一个挑战。在这里,我们提出了一种通过自组装核心、疏水基序和细胞渗透单元的嵌合来开发具有双重功能的自组装纳米肽的策略。优选的纳米肽F3FT和N3FT具有较强的抗菌活性和良好的生物相容性。至关重要的是,F3FT和N3FT能够有效地穿透细胞,消除细胞内细菌和狙击炎症。此外,F3FT和N3FT主要通过破坏细菌细胞膜和诱导活性氧的过度积累来杀死细菌。F3FT和N3FT在体内已显示出良好的安全性和强大的治疗潜力。这种通过多功能结构域设计构建纳米肽的方案为处理细胞内细菌和抗菌素耐药性的升级提供了一种范例。本文章由计算机程序翻译,如有差异,请以英文原文为准。
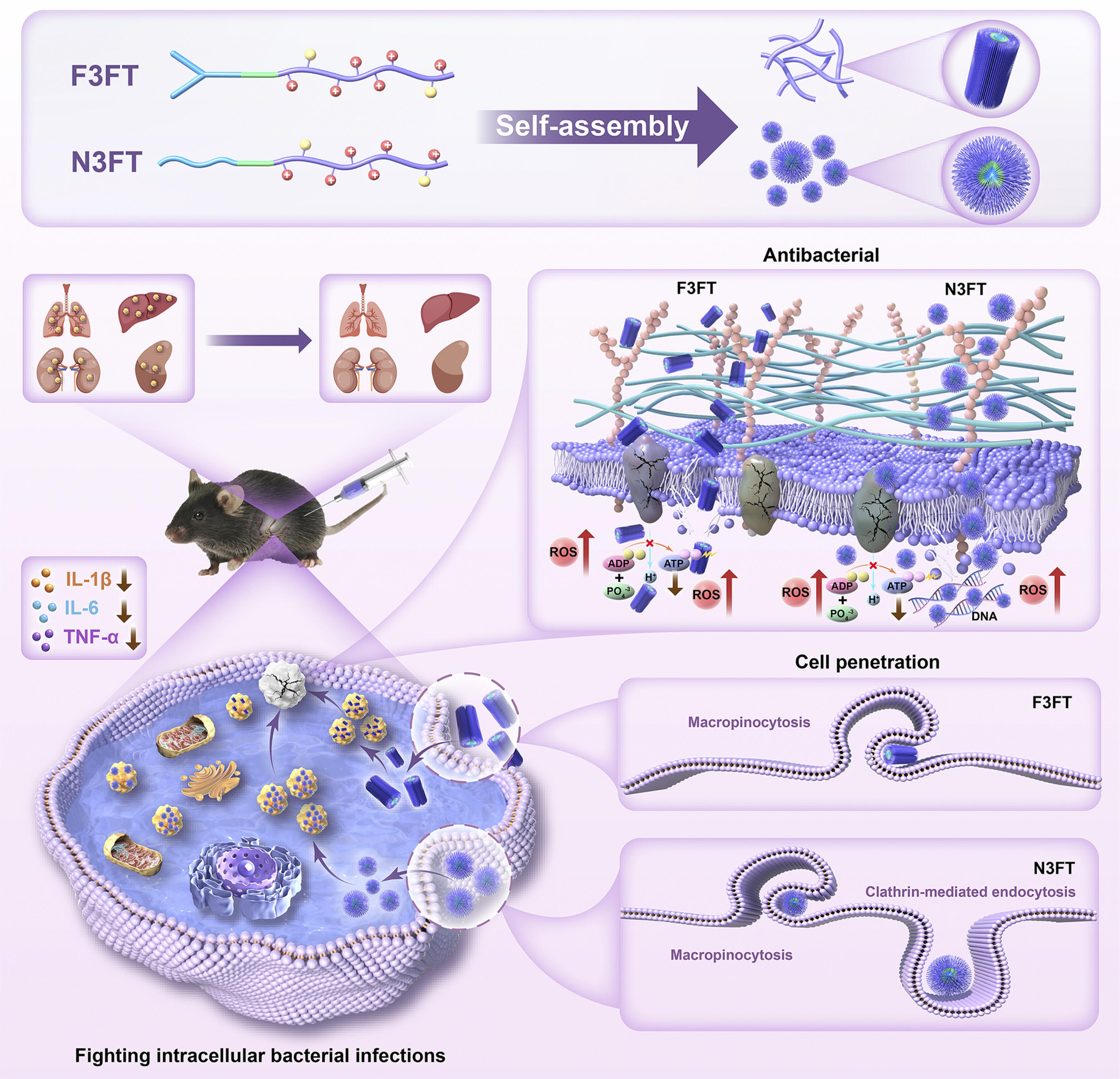
Self-assembling peptide with dual function of cell penetration and antibacterial as a nano weapon to combat intracellular bacteria
Intracellular bacterial infections and antimicrobial resistance are threatening global public health systems. Antimicrobial peptides are a potential solution to combat bacterial resistance, but the design of self-assembled nanopeptides with dual functions of cell penetration and antibacterial properties to combat intracellular bacteria remains a challenge. Here, we propose a strategy to develop self-assembled nanopeptides with dual functions through the chimerization of self-assembled core, hydrophobic motif, and cell-permeable unit. The optimal nanopeptides, F3FT and N3FT, exhibited potent antibacterial activity and excellent biocompatibility. Crucially, F3FT and N3FT are able to efficiently penetrate cells and eliminate intracellular bacteria and sniping inflammation. Moreover, F3FT and N3FT kill bacteria mainly by disrupting bacterial cell membranes and inducing excessive accumulation of reactive oxygen species. F3FT and N3FT have exhibited good safety and potent therapeutic potential in vivo. This scheme of constructing nanopeptides through multifunctional domains design provides a paradigm for dealing with escalating of intracellular bacteria and antimicrobial resistance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


