抗体预防可以掩盖猕猴的亚临床SIV感染
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
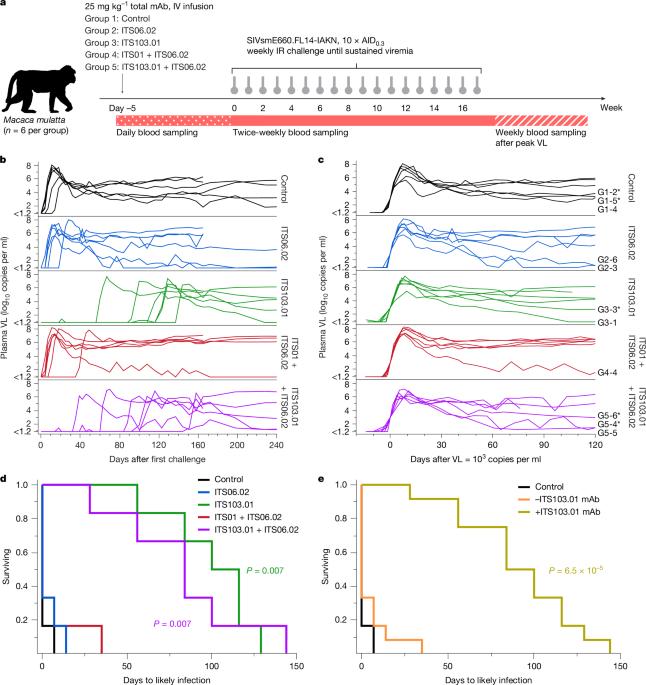
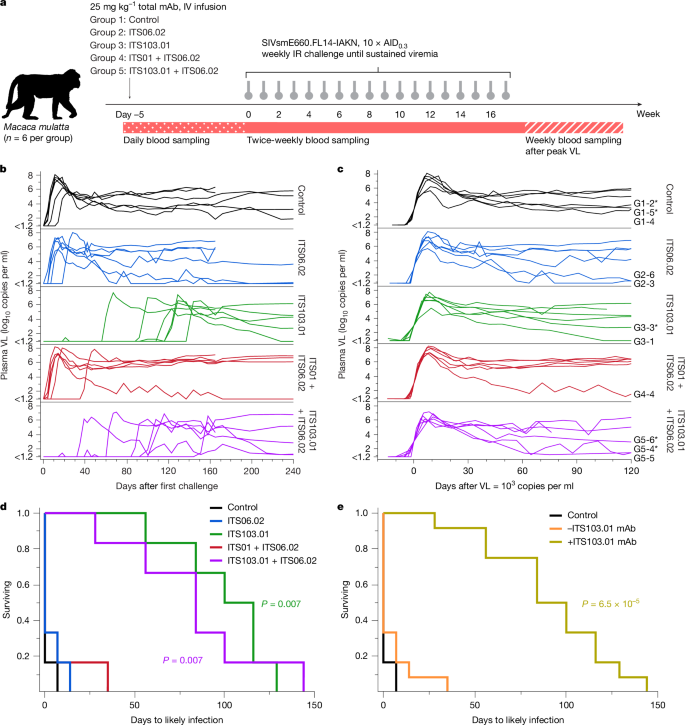
广泛中和抗体(bNAbs)显示出预防人类免疫缺陷病毒(HIV-1)感染的潜力1。然而,关于预防感染所需的抗体浓度的数据有限。bNAb预防的临床试验已经显示出部分效果,但采样频率通常不允许感染事件和并发抗体水平的精确时间。在这里,我们使用猴免疫缺陷病毒(SIV)感染恒河猴,我们发现尽管强效的bNAb可以延缓急性病毒血症的发作,但当bNAb水平保持高水平时,亚临床感染就会发生。对给予部分中和和完全中和的bNAb的猴子进行的SIV连续攻击显示,当血清bNAb浓度远高于目前接受的保护水平时,经常发生“病毒突变”(低且短暂的血浆病毒血症)。为了了解导致这种突变的感染的确切时间,我们对在bNAb给药后连续受到基因条形码SIV攻击的猴子进行了血浆病毒测序。这些分析表明,在给予有效的bNAb预防的大多数动物中发生亚临床感染。这些亚临床感染发生时,抗体浓度比预防完全病毒突破感染所需的水平高2至400倍。本研究表明免疫预防可以掩盖亚临床感染,这可能会影响预防性HIV-1 bNAb临床试验的解释。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Antibody prophylaxis may mask subclinical SIV infections in macaques
Broadly neutralizing antibodies (bNAbs) show potential to prevent human immunodeficiency virus (HIV-1) infection in humans1. However, there are limited data on the antibody concentrations required to prevent infection. Clinical trials of bNAb prophylaxis have demonstrated partial efficacy2, but the sampling frequency typically does not allow precise timing of infection events and concurrent antibody levels. Here, using simian immunodeficiency virus (SIV) infection of rhesus macaques, we show that although potent bNAbs can delay the onset of acute viremia, subclinical infections occur while bNAb levels remain high. Serial SIV challenge of monkeys given partially and fully neutralizing bNAbs revealed that ‘viral blips’—low and transient plasma viremia—often occur while serum bNAb concentrations are well above currently accepted protective levels. To understand the precise timing of the infections resulting in such blips, we performed plasma viral sequencing on monkeys that were serially challenged with genetically barcoded SIV after bNAb administration. These analyses showed that subclinical infections occurred in most animals that were given potent bNAb prophylaxis. These subclinical infections occurred while antibody concentrations were 2- to 400-fold higher than the levels required to prevent fully viremic breakthrough infection. This study demonstrates that immunoprophylaxis can mask subclinical infections, which may affect the interpretation of prophylactic HIV-1 bNAb clinical trials. Viral sequencing reveals subclinical infections in animals challenged with simian immunodeficiency virus even in the presence of potent neutralizing antibodies, with implications for clinical trials of antiviral agents against human immunodeficiency virus.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


