DNA逻辑门触发膜融合准确检测和杀死癌细胞
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
准确、灵敏地识别和杀死癌细胞,特别是深层组织中的癌细胞,是至关重要的,但也面临着重大挑战。本文设计了一种膜蛋白和三磷酸腺苷(ATP)驱动的DNA逻辑门修饰脂质体,将氧化锌(ZP)纳米颗粒与纳米酶(ZP/RuTe@L/DNA)结合,在癌细胞酸性条件下通过活性氧(ROS)介导的机制准确识别和诱导癌细胞凋亡。在这个系统中,DNA逻辑门功能化脂质体装载了ZP和纳米酶,而HeLa癌细胞则用DNA片段功能化,该DNA片段与DNA逻辑门片段互补。对于DNA逻辑门,一个DNA适体用于膜蛋白识别,另一个适体用于细胞外ATP的响应。DNA逻辑门的激活只有在两种生物标志物同时存在时才会发生。DNA逻辑门修饰的脂质体一旦被激活,就可以与DNA片段修饰的HeLa细胞杂交,导致脂质体与HeLa细胞融合,并释放ZP/RuTe到HeLa细胞中。在酸性条件下,ZP可以分解释放H2O2和Zn2+,通过抑制电子传递链促进•O2 -和H2O2的生成。同时,释放的RuTe表现出谷胱甘肽(GSH)耗竭、过氧化物酶(POD)和烟酰胺腺嘌呤二核苷酸(NADH)过氧化物酶样活性,产生高毒性羟基自由基(•OH),破坏细胞氧化还原稳态,诱导细胞凋亡。ZP/RuTe@L/DNA系统不仅可以准确检测复杂细胞混合物中的癌细胞,而且为脂质体-膜融合给药过程提供了一种新的方法。该研究在癌症的精确诊断和治疗中具有重要的应用潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
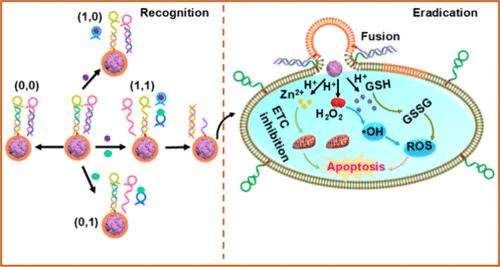
DNA Logic Gate-Triggered Membrane Fusion for Accurately Detecting and Killing Cancer Cells
Accurately and sensitively identifying and killing cancer cells, especially those in deep tissues, is of paramount importance but presents significant challenges. Herein, a membrane protein and adenosine triphosphate (ATP)-driven DNA logic gate-modified liposome is designed to coat zinc peroxide (ZP) nanoparticles integrated with nanozymes (ZP/RuTe@L/DNA) to accurately identify and induce cell apoptosis in cancer cells through a reactive oxygen species (ROS)-mediated mechanism under acid conditions in cancer cells. In this system, DNA logic gate-functionalized liposomes are loaded with ZP and nanozymes, while HeLa cancer cells are functionalized with a DNA segment that is complementary to a segment of the DNA logic gate. For the DNA logic gate, a DNA aptamer was employed for membrane protein recognition, and another aptamer was used for the response of extracellular ATP. Activation of the DNA logic gate occurs only when both biomarkers are simultaneously present. Once activated, the DNA logic gate-modified liposome could hybridize with DNA segment-modified HeLa cells, leading to liposome–HeLa cell fusion and the release of ZP/RuTe into HeLa cells. Under acid conditions, ZP could decompose to release H2O2 and Zn2+, which could promote the production of •O2– and H2O2 by inhibiting the electron transport chain. Concurrently, the released RuTe exhibits glutathione (GSH) depletion and peroxidase (POD) and nicotinamide adenine dinucleotide (NADH) peroxidase-like activities, generating highly toxic hydroxyl radical (•OH), disrupting the cellular redox homeostasis, and inducing cell apoptosis. The ZP/RuTe@L/DNA system could not only accurately detect cancer cells in complex cell mixtures but also present a novel method for liposome–membrane fusion processes in drug delivery. This study presents significant potential for application in precise cancer diagnosis and therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


