dupilumab对慢性鼻窦炎伴鼻息肉的长期影响:迈向临床缓解的一步。
IF 4.3
2区 医学
Q2 ALLERGY
引用次数: 0
摘要
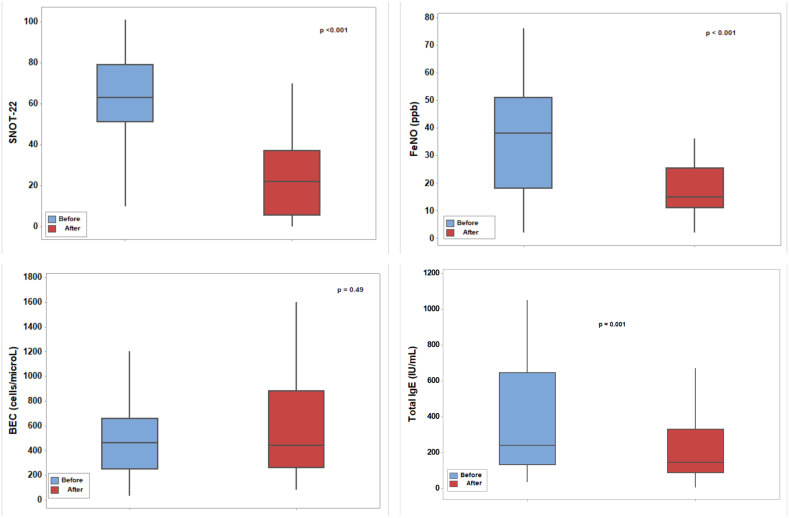
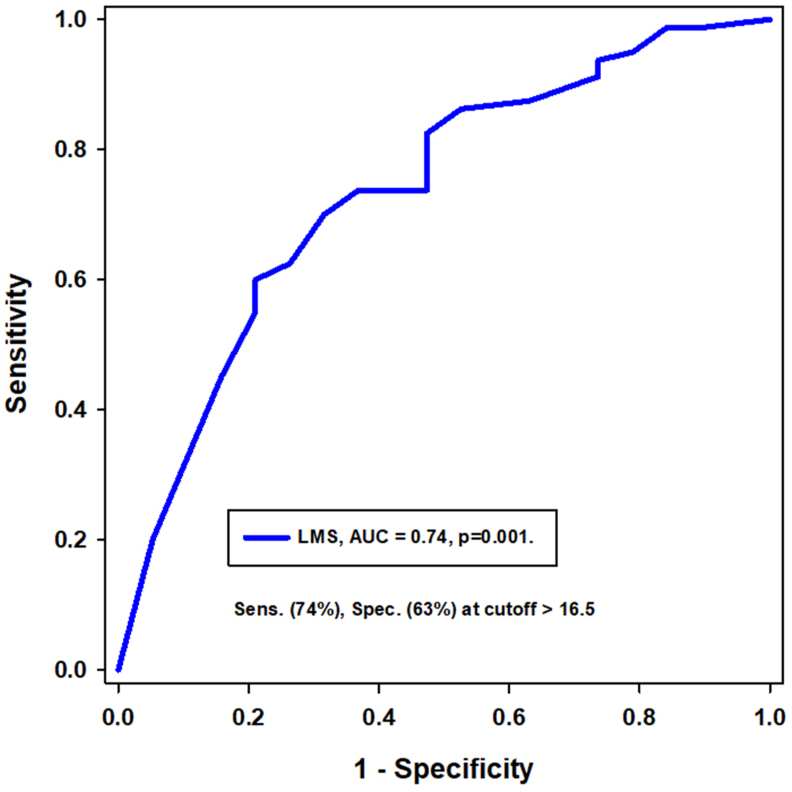
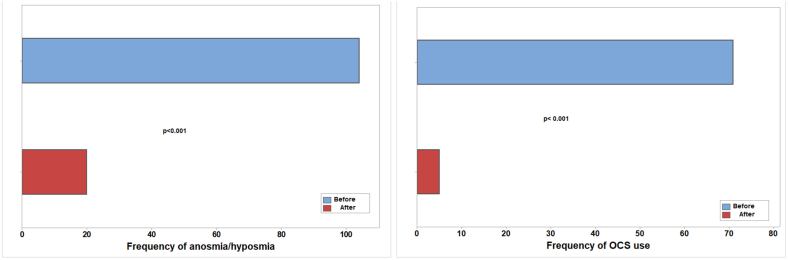
背景和目的:临床缓解,定义为疾病活动和症状的消失,是慢性鼻窦炎伴鼻息肉(CRSwNP)治疗的一个新兴目标。本研究旨在评估dupilumab对伴有或不伴有哮喘的CRSwNP患者的长期影响,并探索实现临床缓解的潜力。方法:对109例伴有或不伴有哮喘的CRSwNP患者进行了一项为期两年的前瞻性研究,这些患者符合dupilumab作为附加治疗的条件。在治疗前后进行综合评估,包括临床、实验室和放射学评估。CRSwNP的临床缓解定义为dupilumab治疗12个月,无需要口服皮质类固醇(OCS)的恶化,不需要鼻窦手术,无嗅觉缺失或低通气,鼻-鼻预后测试(SNOT-22)评分低于20,lnd - mackay评分低于10。对于合并哮喘的患者,临床缓解的定义是哮喘控制测试(ACT)得分为19分或更高,没有哮喘加重,不需要OCS。结果:Dupilumab显著改善了两组患者的CRSwNP结果,包括SNOT-22评分、鼻息肉大小(LMS)和嗅觉缺失/低血症。共病哮喘非常普遍(79.8%),哮喘患者在dupilumab治疗前后鼻息肉明显增大,尽管症状改善相似。较高的呼出一氧化氮(FeNO)分数和血嗜酸性粒细胞计数(BEC)水平,以及嗅觉缺失/低血症,预示着更大的息肉大小。Dupilumab还显著改善哮喘结局,增加1s用力呼气量(FEV1)并降低FeNO。11%的患者达到临床缓解,哮喘患者的比例略低(7.3%)。结论:Dupilumab治疗CRSwNP可达到临床缓解。然而,合并症哮喘似乎降低了缓解的可能性,并且与更大的鼻息肉相关,即使症状改善相似。哮喘可能独立影响息肉的发育,潜在地影响CRSwNP的长期预后。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Long-term effects of dupilumab on chronic rhinosinusitis with nasal polyps: A step towards clinical remission
Background and objectives
Clinical remission, defined as the absence of disease activity and symptoms, is an emerging goal in the management of chronic rhinosinusitis with nasal polyps (CRSwNP). This study aimed to evaluate the long-term effects of dupilumab on patients with CRSwNP, with or without asthma, and explore the potential for achieving clinical remission.
Methods
A two-year prospective study was conducted on 109 patients with CRSwNP, with or without asthma, who were eligible for dupilumab as an add-on therapy. Comprehensive assessments, including clinical, laboratory, and radiological evaluations, were performed before and after treatment. Clinical remission of CRSwNP was defined as 12 months of dupilumab treatment, no exacerbations requiring oral corticosteroids (OCS), no need for nasal sinus operation, no anosmia or hyposmia, a Sino-Nasal Outcome Test (SNOT-22) score under 20, and a Lund-Mackay score (LMS) below 10. For those with comorbid asthma, clinical remission was defined as an asthma control test (ACT) score of 19 or higher, no asthma exacerbations, and no need for OCS.
Results
Dupilumab significantly improved CRSwNP outcomes in both groups, including SNOT-22 scores, nasal polyp size (LMS), and anosmia/hyposmia. Comorbid asthma was highly prevalent (79.8%), and patients with asthma had significantly larger nasal polyps, both before and after dupilumab therapy, despite similar symptom improvement. Higher fractional exhaled nitric oxide (FeNO) and blood eosinophil count (BEC) levels, along with anosmia/hyposmia, predicted larger polyp size. Dupilumab also significantly improved asthma outcomes, increasing forced expiratory volume in 1 s (FEV1) and decreasing FeNO. Clinical remission was achieved in 11% of patients, with a slightly lower rate in those with asthma (7.3%).
Conclusion
Dupilumab treatment can achieve clinical remission in CRSwNP. However, comorbid asthma appears to reduce the likelihood of remission and is associated with larger nasal polyps, even with similar symptom improvement. Asthma may independently influence polyp development, potentially impacting long-term outcomes in CRSwNP.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

World Allergy Organization Journal
Immunology and Microbiology-Immunology
CiteScore
9.10
自引率
5.90%
发文量
91
审稿时长
9 weeks
期刊介绍:
The official pubication of the World Allergy Organization, the World Allergy Organization Journal (WAOjournal) publishes original mechanistic, translational, and clinical research on the topics of allergy, asthma, anaphylaxis, and clincial immunology, as well as reviews, guidelines, and position papers that contribute to the improvement of patient care. WAOjournal publishes research on the growth of allergy prevalence within the scope of single countries, country comparisons, and practical global issues and regulations, or threats to the allergy specialty. The Journal invites the submissions of all authors interested in publishing on current global problems in allergy, asthma, anaphylaxis, and immunology. Of particular interest are the immunological consequences of climate change and the subsequent systematic transformations in food habits and their consequences for the allergy/immunology discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


