造血干细胞移植方案对他克莫司药代动力学的影响。
IF 1.5
Q3 MEDICINE, RESEARCH & EXPERIMENTAL
Current Therapeutic Research-clinical and Experimental
Pub Date : 2025-01-01
DOI:10.1016/j.curtheres.2024.100775
引用次数: 0
摘要
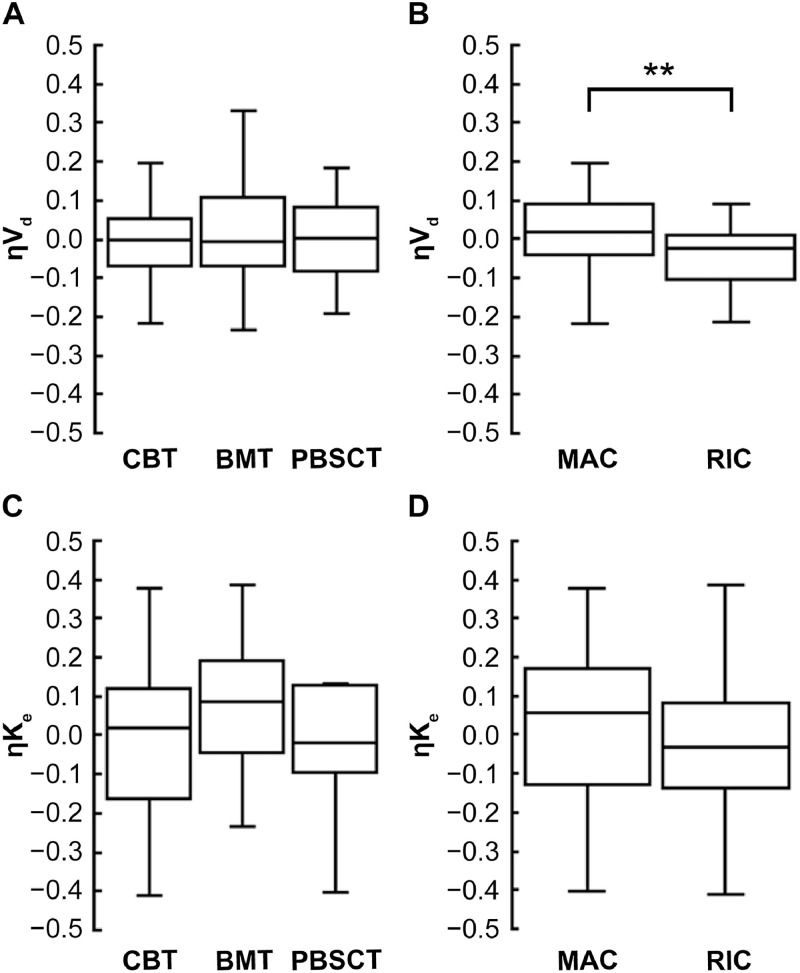
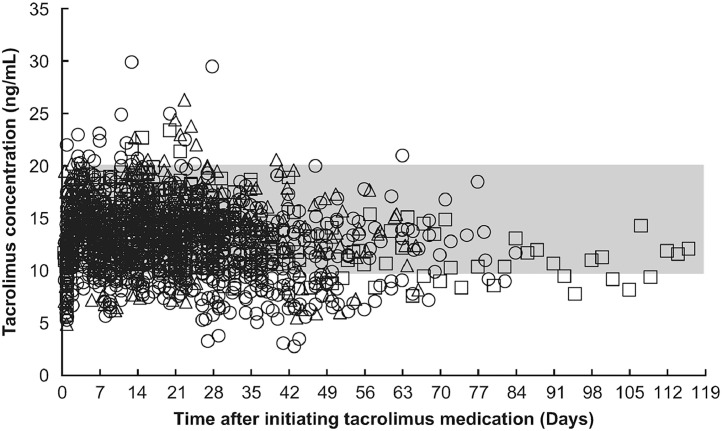
目的:他克莫司治疗需要严格控制异基因造血干细胞移植(HSCT)患者的全血浓度。在接受脐带血移植(CBT)的患者中,他克莫司的分布容积与反映红细胞(RBC)计数的血红蛋白水平呈负相关。在这项研究中,我们评估了调节方案(清髓和低强度调节)或供体来源(脐带血、骨髓和外周血干细胞)对他克莫司在接受HSCT患者(包括接受CBT的患者)中的药代动力学的影响。我们还研究了考虑红细胞计数的他克莫司给药策略的适用性。方法:我们回顾性分析了HSCT患者的临床资料,包括他克莫司全血浓度。观察期从第一次连续静脉输注到改用口服药物、转院、复发或死亡。对观察期间治疗药物监测所得的他克莫司全血浓度进行群体药动学分析。患者特征和实验室数据作为协变量进行评估。结果:我们招募了91例接受HSCT的患者(CBT: n = 56;骨髓移植:n = 22;外周血干细胞移植:n = 13);58例和33例患者分别接受清骨髓调节和降低强度调节。使用单室加性误差模型准确捕获全血他克莫司浓度(n = 1,658个测量值)。调理方案和供体来源对他克莫司的药代动力学没有影响。因此,在制定给药策略时没有考虑这些因素。然而,体积分布和血红蛋白水平之间的负相关被证实,这表明监测红细胞计数对评估给药策略是有用的。结论:考虑血红蛋白水平变化的他克莫司给药策略适用于所有接受造血干细胞移植的患者。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Effect of Hematopoietic Stem Cell Transplantation Regimen on Tacrolimus Pharmacokinetics
Objectives
Treatment with tacrolimus requires strict control of the whole-blood concentration in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT). In patients undergoing cord blood transplantation (CBT), there is a negative correlation between volume of distribution of tacrolimus and hemoglobin levels, which reflect the red blood cell (RBC) count. In this study, we evaluated the influence of the conditioning regimen (myeloablative and reduced-intensity conditioning) or donor source (cord blood, bone marrow, and peripheral blood stem cells) on the pharmacokinetics of tacrolimus in patients undergoing HSCT, including those undergoing CBT. We also examined applicability of dosing strategy of tacrolimus considering the RBC count.
Methods
We retrospectively analyzed clinical data—including whole-blood tacrolimus concentrations—from patients with HSCT. The observation period spanned from first continuous intravenous infusions until switch to oral medication, transfer to another hospital, relapse, or death. Population pharmacokinetic analysis was performed on whole-blood tacrolimus concentrations obtained from therapeutic drug monitoring during the observation period. Patient characteristics and laboratory data were evaluated as covariates.
Results
We enrolled 91 patients undergoing HSCT (CBT: n = 56; bone marrow transplantation: n = 22; and peripheral blood stem cell transplantation: n = 13); 58 and 33 patients received myeloablative conditioning and reduced-intensity conditioning, respectively. Whole-blood tacrolimus concentrations were accurately captured (n = 1,658 measurements) using a one-compartment and additive error model. The conditioning regimen and donor source did not have an impact on the pharmacokinetics of tacrolimus. Therefore, these factors were not considered when forming the dosing strategy. Nevertheless, a negative correlation between volume of distribution and hemoglobin level was confirmed, indicating that monitoring the RBC count is useful in assessing the dosing strategy.
Conclusions
A tacrolimus dosing strategy that considers the variability in hemoglobin levels applies to all patients undergoing HSCT.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
0.00%
发文量
31
审稿时长
3 months
期刊介绍:
We also encourage the submission of manuscripts presenting preclinical and very preliminary research that may stimulate further investigation of potentially relevant findings, as well as in-depth review articles on specific therapies or disease states, and applied health delivery or pharmacoeconomics.
CTR encourages and supports the submission of manuscripts describing:
• Interventions designed to understand or improve human health, disease treatment or disease prevention;
• Studies that focus on problems that are uncommon in resource-rich countries;
• Research that is "under-published" because of limited access to monetary resources such as English language support and Open Access fees (CTR offers deeply discounted English language editing);
• Republication of articles previously published in non-English journals (eg, evidence-based guidelines) which could be useful if translated into English;
• Preclinical and clinical product development studies that are not pursued for further investigation based upon early phase results.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


