沸石中葡萄糖合成葡聚糖低聚物的限制性研究
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
葡聚糖低聚物是一种独特的葡萄糖衍生成分,基于其聚合度(DP)和糖苷键种类,作为植物生长促进剂和益生元具有广泛的应用。然而,在转换水介质中,葡萄糖上的5个游离-OH基团之间可以进行糖基化,导致葡聚糖低聚物通过各种不受控制的糖苷键连接,这可能导致其应用的不确定性。为了解决这一挑战,开发了在沸石微孔中限制合成葡聚糖低聚物的方法。通过FAU框架或MOR框架将葡萄糖浸渍到H-Y的微孔通道中,限制其空间构型,从而选择性地产生具有特定糖苷键的葡聚糖低聚物。结果表明,在H-Y反应中,生成的葡聚糖低聚物的产率为41.7%,DP值为2 ~ 5,形成的葡聚糖低聚物中有59.2%通过(1→6)糖苷键连接。当H-MOR作为催化剂时,葡萄糖单元通过87.6%的(1→4)键连接,获得了61.7%的高葡聚糖低聚物收率。MOR沸石的一维微孔结构不仅有利于控制糖苷键结构,而且可以提高所产生的葡聚糖低聚物的扩散,使葡聚糖低聚物易于从沸石中分离出来。用于糖基化的H-Y和H-MOR在去除有机残留物后,可在空气中煅烧再生,催化剂可循环使用5次以上,且催化性能不下降。本文章由计算机程序翻译,如有差异,请以英文原文为准。
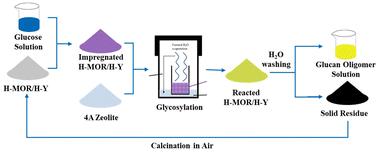
Confined synthesis of glucan oligomers from glucose in zeolites†
Glucan oligomer is a unique glucose-derived component with versatile applications as a plant growth elicitor and prebiotic based on its degree of polymerization (DP) and glycosidic bond species. However, in conversional aqueous media, glycosylation can be carried out among 5 free –OH groups on glucose, resulting in a glucan oligomer linked through various uncontrolled glycosidic bonds, which can lead to uncertainty of their application. To address this challenge, the confined synthesis of the glucan oligomer in the micropores of zeolites was developed. Glucose was impregnated into the microporous channel of H-Y with the FAU framework or H-MOR with the MOR framework to restrict its spatial configuration and thereby selectively produce the glucan oligomer with specific glycosidic bonds. The results showed that a 41.7% yield of the glucan oligomer with the DP ranging from 2 to 5 can be produced in H-Y, and 59.2% of the formed glucan oligomer was linked through (1 → 6) glycosidic bonds. When H-MOR was used as a catalyst, a high glucan oligomer yield of 61.7% was achieved with glucose units linked through 87.6% of (1 → 4) linkages. The one-dimensional microporous structure of MOR zeolites is not only beneficial for controlling the glycosidic bond structure, but can also improve the diffusion of the produced glucan oligomer, resulting in facile separation of the glucan oligomer from the zeolites. The H-Y and H-MOR used in glycosylation can be readily regenerated via calcination in air after removing the organic residues, and the catalysts can be recycled more than 5 times without catalytic performance decline.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


