光致电子转移与氢原子转移的合并:羰基的形式β-C(sp3) -H吡啶化
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-01-09
DOI:10.1021/acs.joc.4c0273910.1021/acs.joc.4c02739
引用次数: 0
摘要
本研究提出了一种结合光诱导电子转移(ET)和氢原子转移(HAT)的羰基化合物选择性β-C(sp3) -H吡啶化的新方法。这种方法值得注意的是,它不含过渡金属,并且能够在良性反应条件下发挥作用,从而产生一系列的吡啶羰基衍生物,收率始终中等到良好。该技术的意义进一步强调了其在药学上重要分子的后期功能化的潜力。机理研究证实该反应是通过自由基介导的途径进行的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
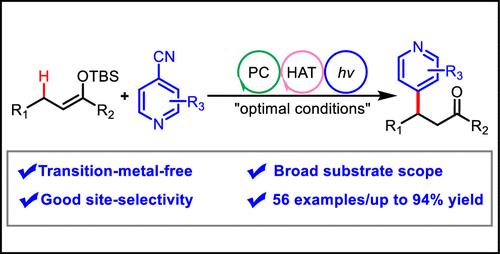
Merging Photoinduced Electron Transfer with Hydrogen Atom Transfer: Formal β-C(sp3)–H Pyridination of Carbonyls
In this study, a novel approach that combines photoinduced electron transfer (ET) with hydrogen atom transfer (HAT) has been introduced for the selective β-C(sp3)–H pyridination of carbonyl compounds. This method is notable for its absence of transition metals and its ability to function under benign reaction conditions, resulting in a range of pyridinated carbonyl derivatives with consistently moderate to good yields. The significance of this technique is further underscored by its potential for the late-stage functionalization of pharmaceutically significant molecules. Mechanistic investigations confirmed that the reaction proceeds via a radical-mediated pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


