铜催化环化合成杂原子包埋的9元环炔和6元硫丹
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-01-06
DOI:10.1021/acs.joc.4c0193510.1021/acs.joc.4c01935
引用次数: 0
摘要
在温和条件下,建立了铜催化、碱控制的简易环化反应,合成了9元环炔和6元杂环磺胺。该方案利用铜催化的分子内A3(炔-醛-胺)偶联反应,有效地合成了9元环炔磺胺,收率高达90%。或者,通过用DBU取代NaHCO3,该方案实现了n -丙炔基的选择性脱保护,从而促进了6元杂环磺胺的形成,收率也很高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
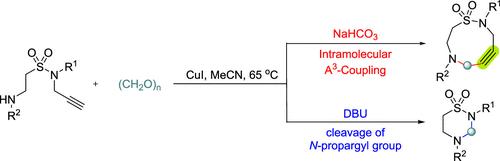
Base-Controlled Synthesis of Heteroatom-Embedded 9-Membered Cycloalkynes and 6-Membered Sultams through Copper-Catalyzed Cyclization
A facile copper-catalyzed, base-controlled cyclization reaction has been developed for the synthesis of 9-membered cycloalkyne and 6-membered heterocycle sultams under mild conditions. This protocol utilizes a copper-catalyzed intramolecular A3 (alkyne–aldehyde–amine) coupling reaction to efficiently synthesize 9-membered cycloalkyne sultams in yields up to 90%. Alternatively, by substituting NaHCO3 with DBU, the protocol achieves selective deprotection of the N-propargyl group, thereby facilitating the formation of 6-membered heterocyclic sultams, also in high yields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


