氢过硫化物(RSSH)介导的烯烃氢硫基化反应
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-01-03
DOI:10.1021/acs.joc.4c0230910.1021/acs.joc.4c02309
引用次数: 0
摘要
建立了一种氢过硫化物介导的烯烃氢硫基化反应,以马尔可夫尼科夫选择性形成C-S键。这种新方法是在温和的条件下进行过渡金属、添加剂和无溶剂的反应。根据我们的控制实验和密度泛函理论计算,假设该反应以离子机制进行,并释放单质硫。本文章由计算机程序翻译,如有差异,请以英文原文为准。
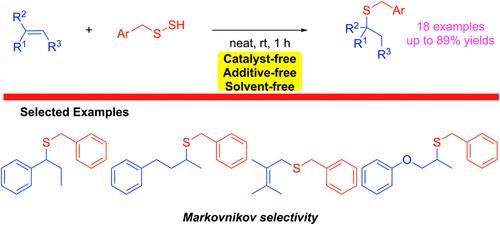
Hydropersulfide (RSSH)-Mediated Hydrothiolation of Alkenes
A hydropersulfide-mediated hydrothiolation reaction of alkenes has been developed for C–S bond formation with Markovnikov selectivity. This new approach is a transition-metal-, additive-, and solvent-free reaction under mild conditions. The reaction is postulated to proceed by an ionic mechanism with the release of elemental sulfur based on our control experiments and density functional theory calculations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


