可见光诱导自扩散自由基反应合成酯类化合物
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-01-16
DOI:10.1021/acs.joc.4c0266210.1021/acs.joc.4c02662
引用次数: 0
摘要
我们在此公开了一种用BrCCl3通过醛与酚的交叉脱氢偶联在可见光诱导下合成邻芳基酯的方法,其中酚酸盐同时作为底物和光敏剂。这种无过渡金属和光催化剂的可见光诱导酯化反应适用于各种底物,产率中等至优异(高达95%)。机理研究提供了自传播的自由基反应的证据,包括醛基C-H键的均裂裂解和酰基溴的形成。BrCCl3作为氧化剂和氢原子转移(HAT)剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
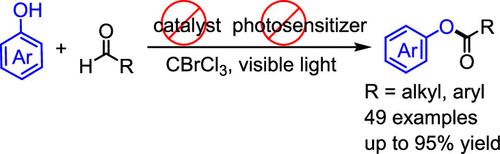
Visible-Light-Induced Synthesis of Esters via a Self-Propagating Radical Reaction
We herein disclose a visible-light-induced synthesis of O-aryl esters through the cross-dehydrogenative coupling of aldehydes with phenols using BrCCl3, in which phenolate functions as both a substrate and a photosensitizer. This transition-metal- and photocatalyst-free visible-light-induced esterification is suitable for a wide range of substrates and gives moderate to excellent yields (up to 95%). Mechanistic studies provided evidence of a self-propagating radical reaction involving homolytic cleavage of the aldehydic C–H bond and the formation of acyl bromides. BrCCl3 serves as an oxidant and a hydrogen atom transfer (HAT) agent.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


