双光氧化还原和铜催化羟基肟酸衍生物与甘氨酸衍生物的不对称远端C(sp3) -H烷基化反应
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-01-14
DOI:10.1021/acs.joc.4c0167710.1021/acs.joc.4c01677
引用次数: 0
摘要
通过1,5-氢转移(1,5- hat)过程实现了双光氧化还原和铜催化的羟基肟酸衍生物与甘氨酸衍生物的远端不对称C(sp) 3-H烷基化反应。该反应具有氧化还原中性、反应条件温和、产率高、对映选择性好、底物范围广等特点。该方案提供了一个直接和有效的策略,以制备高价值的对映体富集的非典型α-氨基酸。此外,该反应的潜在合成价值在高非对映比的二肽的后期不对称烷基化中得到了证明。本文章由计算机程序翻译,如有差异,请以英文原文为准。
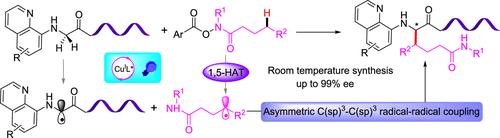
Dual Photoredox and Copper-Catalyzed Asymmetric Remote C(sp3)–H Alkylation of Hydroxamic Acid Derivatives with Glycine Derivatives
Dual photoredox and copper-catalyzed remote asymmetric C(sp)3–H alkylation of hydroxamic acid derivatives with glycine derivatives via a 1,5-hydrogen transfer (1,5-HAT) process has been realized. The reaction was characterized by redox-neutral and mild conditions, good yields, excellent enantioselectivity, and broad substrate scope. This protocol provides a straightforward and efficient strategy to prepare highly valuable enantioenriched noncanonical α-amino acids. Moreover, the potential synthetic value of this reaction was demonstrated in late-stage asymmetric alkylation of dipeptides with a high diastereomeric ratio.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


