Pd(II)催化脱氢环化1-(2-氨基芳基)-3-芳基丙烷-1- ones一步合成2-芳基喹啉-4(1H)- ones
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-12-31
DOI:10.1021/acs.joc.4c0190110.1021/acs.joc.4c01901
引用次数: 0
摘要
在这项研究中,我们开发了钯催化脱氢环化,以原子经济的方式将1-(2-氨基芳基)-3-芳基丙烷-1- 1转化为2-芳基喹啉-4(1H)- 1,也称为aza-flavones,是黄酮的生物异构体。该方法与广泛的底物范围具有良好的化学相容性,可容纳多达25个衍生物。此外,还进行了动力学研究以阐明反应机理。此外,2-苯基喹啉-4(1H)- 1被用作合成4-甲氧基喹啉、n-甲基喹啉-4(1H)- 1和4-(伪)卤化喹啉等特殊结构的常见中间体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
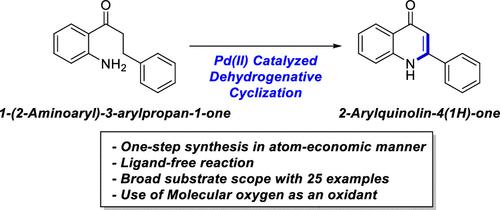
One-Step Synthesis of 2-Arylquinolin-4(1H)-Ones from 1-(2-Aminoaryl)-3-Arylpropan-1-Ones via Pd(II)-Catalyzed Dehydrogenative Cyclization
In this study, we developed palladium-catalyzed dehydrogenative cyclization to transform 1-(2-aminoaryl)-3-arylpropan-1-ones into 2-arylquinolin-4(1H)-ones, also known as aza-flavones which are the bioisosteres of flavones, in an atom-economic manner. This method exhibited excellent chemical compatibility with a broad substrate scope, accommodating up to 25 derivatives. Additionally, kinetic studies were performed to elucidate the reaction mechanism. Further, 2-phenylquinolin-4(1H)-one was used as a common intermediate for synthesizing privileged structures such as 4-methoxyquinoline, N-methylquinoline-4(1H)-one, and 4-(pseudo)halogenated quinoline moieties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


