扩大RNAi治疗的共轭空间:反义链3 '端的配体获得了不受影响的体内效力和功效,并揭示了与Argonaute-2 PAZ结构域的相互作用
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
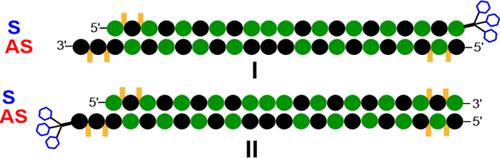
小干扰RNA (siRNA)的传感链与肝细胞特异性受体的配体三n -乙酰半乳糖胺(GalNAc)结合,使多种临床批准的治疗药物能够通过RNA干扰途径发挥作用。在这里,我们首次报道了对反义链3 '端与GalNAc偶联的sirna的系统评价。这些设计保留了相同的受体亲和力,体外和体内活性,以及与具有galnac共轭意义链的sirna相同水平的负载到rna诱导的沉默复合物中。具有22个核苷酸的galnac -共轭反义链的siRNA比具有23个核苷酸反义链的siRNA具有更好的活性。galnac -共轭反义链与Ago2的PAZ结构域的复合体的计算模型证明了3 '端磷酸与PAZ结构域相互作用的重要性,从而解释了这些sirna的活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Expanding Conjugate Space of RNAi Therapeutics: Ligand at the 3′ End of the Antisense Strand Achieves Uncompromised In Vivo Potency and Efficacy and Reveals Interactions with the Argonaute-2 PAZ Domain
The conjugation of the sense strands of small interfering RNA (siRNA) to tri-N-acetylgalactosamine (GalNAc), the ligand for a hepatocyte-specific receptor, enables the delivery of multiple clinically approved therapeutic agents that act through the RNA interference pathway. Here, we report the systematic evaluation of siRNAs with the 3′ termini of antisense strands conjugated to GalNAc for the first time. These designs retained the same receptor affinity, in vitro and in vivo activities, as well as the same level of loading into the RNA-induced silencing complex as siRNAs with a GalNAc-conjugated sense strand. A siRNA with a GalNAc-conjugated antisense strand of 22 nucleotides had better activity than a siRNA with a 23-nucleotide antisense strand. Computational modeling of a complex of a GalNAc-conjugated antisense strand with the PAZ domain of Ago2 rationalizes the importance of the interaction of phosphate at the 3′ terminus with the PAZ domain to explain the observed activity of these siRNAs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


