优化肿瘤内分布的纳米生物杂交溶瘤菌用于声光动力/免疫联合治疗
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
“活体治疗载体”为癌症研究提供了一条很有前途的途径,但由于肿瘤微环境的异质性,在整个实体瘤中实现有效载荷的均匀和持久分布仍然具有挑战性。本研究通过在代谢寡糖工程溶瘤菌YB1表面挂载酸/酶触发的可分离纳米颗粒(HCNs)背包,构建了一个活体药物点缀型生物杂化体(YB1 - HCNs)。在细菌缺氧导航穿透肿瘤的过程中,YB1-HCNs响应性地持续释放HCNs,使载药剂在整个肿瘤中均匀分布。在激光和超声(US)的连续照射下,HCNs可以分别产生II型和I型ROS,用于优越的声光动力治疗(SPDT),这使得HCNs与YB1协同作用,在肿瘤的浅表常氧区和深部缺氧区均获得满意的治疗效果。单次给药后,该有效组合实现了98.3%的原发肿瘤抑制率,使小鼠存活时间延长90天,无复发,进一步诱导了强大的免疫记忆效应,完全抑制了治愈小鼠的肿瘤再攻。这种细菌杂交载体能够优化YB1和HCNs的空间分布,为最大化治疗效果和激发持久的抗肿瘤免疫提供了一种创新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
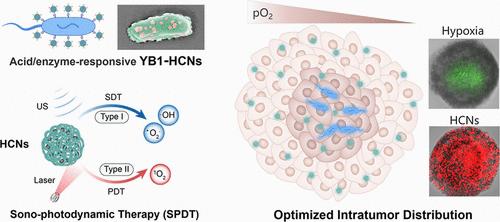
Nanobiohybrid Oncolytic Bacteria with Optimized Intratumoral Distribution for Combined Sono–Photodynamic/Immunotherapy
“Living therapeutic carriers” present a promising avenue for cancer research, but it is still challenging to achieve uniform and durable distribution of payloads throughout the solid tumor owing to the tumor microenvironment heterogeneity. Herein, a living drug sprinkle biohybrid (YB1–HCNs) was constructed by hitching acid/enzyme-triggered detachable nanoparticles (HCNs) backpack on the surface of metabolic oligosaccharide-engineered oncolytic bacteria YB1. Along with the process of tumor penetration by bacterial hypoxia navigation, YB1–HCNs responsively and continuously release HCNs, achieving a uniform distribution of loaded agents throughout the tumor. Upon successive irradiation of laser and ultrasound (US), the HCNs can separately generate type II and type I ROS for superior sono–photodynamic therapy (SPDT), which enables HCNs to synergize with YB1 for a satisfactory therapeutic effect in both superficial normoxic and deep hypoxic regions of the tumor. After a single dose, this efficient combination realized 98.3% primary tumor inhibition rate and prolonged survival of mice for 90 days with no recurrence, further inducing a powerful immunological memory effect to completely suppress tumor rechallenge in cured mice. Such a bacterial hybridization vector enables optimization of the spatial distribution of YB1 and HCNs, providing an innovative strategy to maximize therapeutic outcomes and evoke durable antitumor immunity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


