在固定床反应器中,二氧化钛-1催化剂催化过氧化氢连续合成1,3,2-二氧恶硫烷2,2-二氧化体(DTD
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
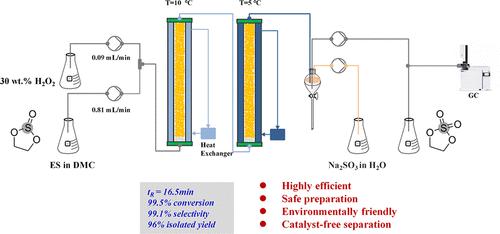
作为锂离子电池的电解质添加剂,1,3,2-二硫代烷2,2-二氧化物(DTD)起着重要的作用。提高电池性能、稳定性和安全性。此外,它是有机化学中常用的羟基化试剂。本文提出了一种高效、安全、环保的不需要催化剂分离的连续DTD合成方法。以球形TS-1为催化剂,H2O2为氧化剂,构建了固定床反应器。在优化的反应条件下,以碳酸二甲酯为溶剂,实现了亚硫酸乙酯(ES)的高效氧化。该过程包括将反应温度分别控制在10°C和5°C的梯度下,保持H2O2与底物的摩尔比为1.05:1,液体每小时空速为0.6 h-1,在底物浓度为1 mol/L的情况下,使用30 wt %浓度的H2O2。转化率达99.5%,选择性为99.1%。为防止DTD水解,反应完成后立即开始连续分离操作,DTD得率达到96%。该工艺的成功应用不仅提高了DTD的生产效率,降低了生产成本,而且为DTD的工业化连续生产奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Continuous Synthesis of 1,3,2-Dioxathiolane 2,2-Dioxide (DTD) by Hydrogen Peroxide with Titanium Silicalite-1 Catalyst Using a Fixed-Bed Reactor
1,3,2-Dioxathiolane 2,2-dioxide (DTD) plays a significant role as an electrolyte additive in lithium-ion batteries. It can enhance battery performance, stability, and safety. Additionally, it is a commonly used hydroxylation reagent in organic chemistry. This paper presents a highly efficient, safe preparation, and environmentally friendly continuous DTD synthesis process that does not require catalyst separation. A fixed-bed reactor was constructed with spherical TS-1 as the catalyst and H2O2 as the oxidizer. Under optimized reaction conditions, efficient oxidation of ethylene sulfite (ES) was achieved using dimethyl carbonate as a solvent. The process involved controlling the reaction temperatures at gradients of 10 and 5 °C, respectively, maintaining a molar ratio of H2O2 to substrate of 1.05:1, a liquid hourly space velocity of 0.6 h–1, and using a 30 wt % concentration of H2O2 at a substrate concentration of 1 mol/L. The conversion rate was up to 99.5%, and the selectivity of DTD was 99.1%. To prevent hydrolysis of DTD, a continuous separation operation was initiated immediately after the completion of the reaction, and the yield of DTD reached 96%. The successful application of this process not only improves the production efficiency of DTD and reduces the production cost but also establishes the foundation for the industrialized continuous production of DTD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


