单宁酸和苯硼酸聚合物自组装的腺相关病毒可躲避中和抗体并减少不良反应
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
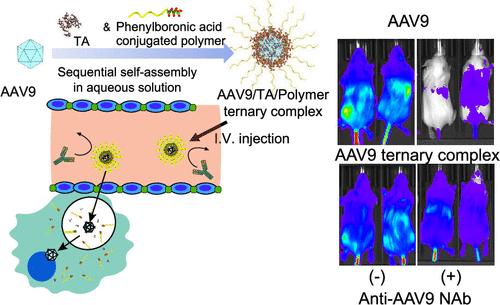
腺相关病毒(aav)越来越多地用于基因治疗,以治疗衰弱性遗传疾病。然而,系统给药的aav通常被中和抗体(nab)灭活,并且大剂量给药的aav会引起肝毒性,从而限制了其有效性。为了解决这些挑战,我们提出了一种基于单宁酸(TA)和苯硼酸共轭聚合物的顺序组装技术,在水溶液中形成具有核壳结构的aav负载三元配合物,其平均直径为60 nm。由于TA包覆AAV并与聚合物上的硼酸形成硼酸酯,AAV血清型9 (AAV9,平均直径为25 nm)被成功包装到一个由聚合物链包围的核心隔室中,形成一个保护壳,以逃避nab的失活。静脉注射的三元复合物成功地避开了nab,并通过减少肝脏积聚来抑制肝毒性。同时,三元复合物通过在细胞内释放AAV9,有效地将基因转导到细胞内,并维持AAV9对靶脑细胞的血脑屏障(BBB)通透性,从而使脑/肝转导选择性比单独使用AAV9提高20倍。此外,将这种装配技术与微泡聚焦超声(MB-FUS)系统相结合,用于无创血脑屏障打开,将其基因转导到大脑的效率提高了6倍以上,并进一步提高了脑/肝转导的选择性。我们的超分子方法结合医疗设备代表了基于aav的基因治疗的重大进步。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Adeno-Associated Virus Self-Assembled with Tannic Acid and Phenylboronic Acid Polymers to Evade Neutralizing Antibodies and Reduce Adverse Events
Adeno-associated viruses (AAVs) are increasingly used in gene therapy to treat debilitating genetic diseases. However, systemically administered AAVs are often inactivated by neutralizing antibodies (NAbs), and high-dose administration of AAVs causes hepatotoxicity, which limits their effectiveness. To address these challenges, we present a sequential assembly technique based on tannic acid (TA) and phenylboronic acid-conjugated polymers to form AAV-loaded ternary complexes having a core–shell structure with an average diameter of 60 nm in an aqueous solution. Since TA coats AAVs and forms boronate esters with boronic acids on polymers, AAV serotype 9 (AAV9, an average diameter of 25 nm) was successfully packaged into a core compartment surrounded by polymer chains forming a protective shell to evade inactivation by NAbs. The intravenously injected ternary complexes successfully evade NAbs and suppress hepatotoxicity by minimizing liver accumulation. Meanwhile, the ternary complex exhibited efficient gene transduction into cells by releasing AAV9 intracellularly and maintained the blood–brain barrier (BBB) permeability of AAV9 to target brain cells, thereby enhancing brain/liver transduction selectivity 20-fold compared to AAV9 alone. Moreover, combining this assembly technique with a microbubble-focused ultrasound (MB-FUS) system for noninvasive BBB opening improves its gene transduction efficiency into the brain by more than 6-fold and further increases brain/liver transduction selectivity. Our supramolecular approach combined with a medical device represents a significant advancement in AAV-based gene therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


