用于催化的封闭水:热力学性质和反应动力学
IF 55.8
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
水是催化系统中的重要成分,在反应中充当反应物、生成物和/或旁观者。在不同的局部环境中,承压水与散装水表现出明显不同的行为。因此,封闭水的广泛化学性质为调整反应动力学提供了巨大的机会。在这篇综述中,我们的重点是建立在非均相(电)催化反应动力学和承压水性质之间的联系。首先,介绍了承压水的性质,其中水的焓、熵和介电性质可以通过调整空腔的几何形状和疏水性来调节。其次,对水与无机材料(如碳纳米管(一维约束)、带电金属或金属氧化物表面(2D)、沸石和金属有机框架(3D)以及离子/溶剂分子(0D))之间相互作用的实验和计算研究进行了回顾,以证明创造具有独特氢键网络性质的约束水结构的机会。第三,讨论了氢键结构和动力学对(电)催化活性自由能、重组能和指前因子的影响。我们强调了通过优化界面氢键网络来调节化学物质和燃料脱碳反应动力学来增强质子耦合电子转移的新机会。本文章由计算机程序翻译,如有差异,请以英文原文为准。
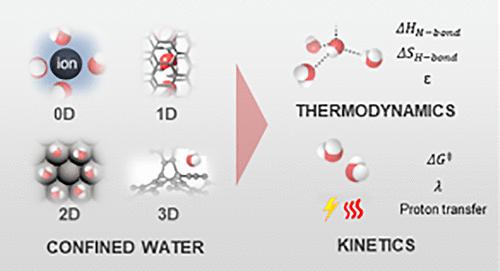
Confined Water for Catalysis: Thermodynamic Properties and Reaction Kinetics
Water is a salient component in catalytic systems and acts as a reactant, product and/or spectator species in the reaction. Confined water in distinct local environments can display significantly different behaviors from that of bulk water. Therefore, the wide-ranging chemistry of confined water can provide tremendous opportunities to tune the reaction kinetics. In this review, we focus on drawing the connection between confined water properties and reaction kinetics for heterogeneous (electro)catalysis. First, the properties of confined water are presented, where the enthalpy, entropy, and dielectric properties of water can be regulated by tuning the geometry and hydrophobicity of the cavities. Second, experimental and computational studies that investigate the interactions between water and inorganic materials, such as carbon nanotubes (1D confinement), charged metal or metal oxide surfaces (2D), zeolites and metal–organic frameworks (3D) and ions/solvent molecules (0D), are reviewed to demonstrate the opportunity to create confined water structures with unique H-bonding network properties. Third, the role of H-bonding structure and dynamics in governing the activation free energy, reorganization energy and pre-exponential factor for (electro)catalysis are discussed. We highlight emerging opportunities to enhance proton-coupled electron transfer by optimizing interfacial H-bond networks to regulate reaction kinetics for the decarbonization of chemicals and fuels.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Reviews
化学-化学综合
CiteScore
106.00
自引率
1.10%
发文量
278
审稿时长
4.3 months
期刊介绍:
Chemical Reviews is a highly regarded and highest-ranked journal covering the general topic of chemistry. Its mission is to provide comprehensive, authoritative, critical, and readable reviews of important recent research in organic, inorganic, physical, analytical, theoretical, and biological chemistry.
Since 1985, Chemical Reviews has also published periodic thematic issues that focus on a single theme or direction of emerging research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


