探索寨卡病毒的动态:通过数学模型从流行病到方程式的范围审查之旅。
IF 2.5
3区 医学
Q1 Medicine
引用次数: 0
摘要
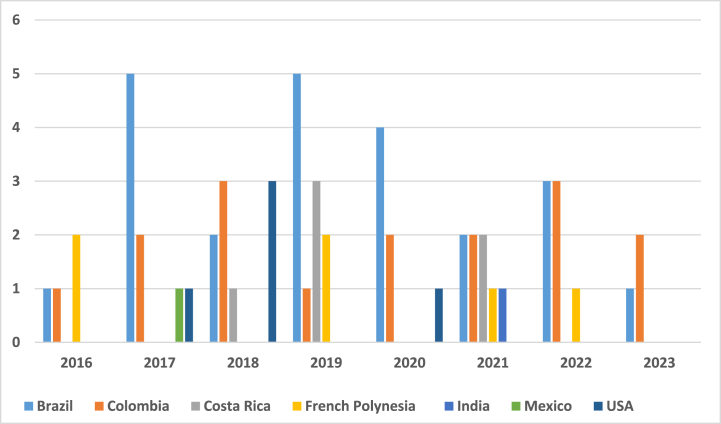
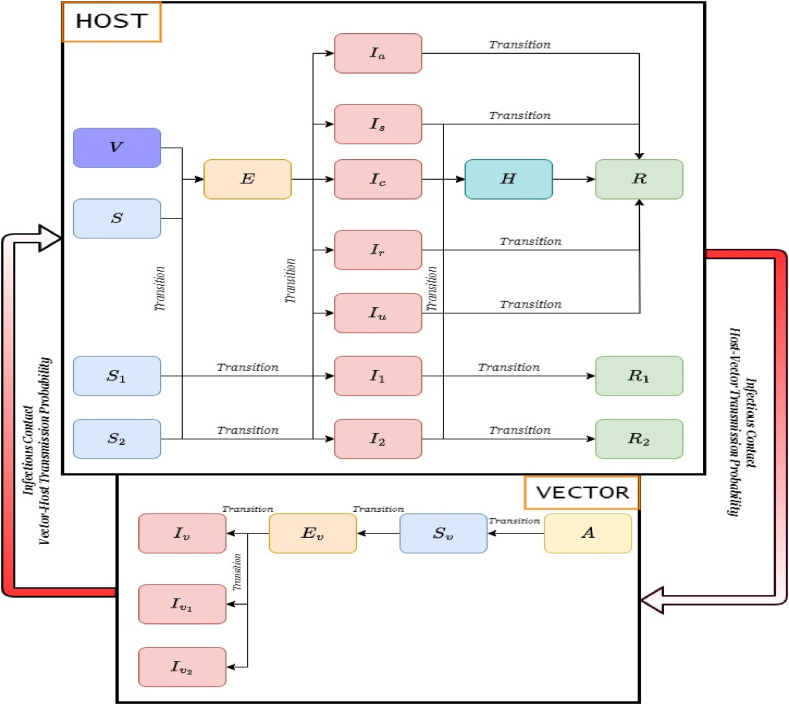
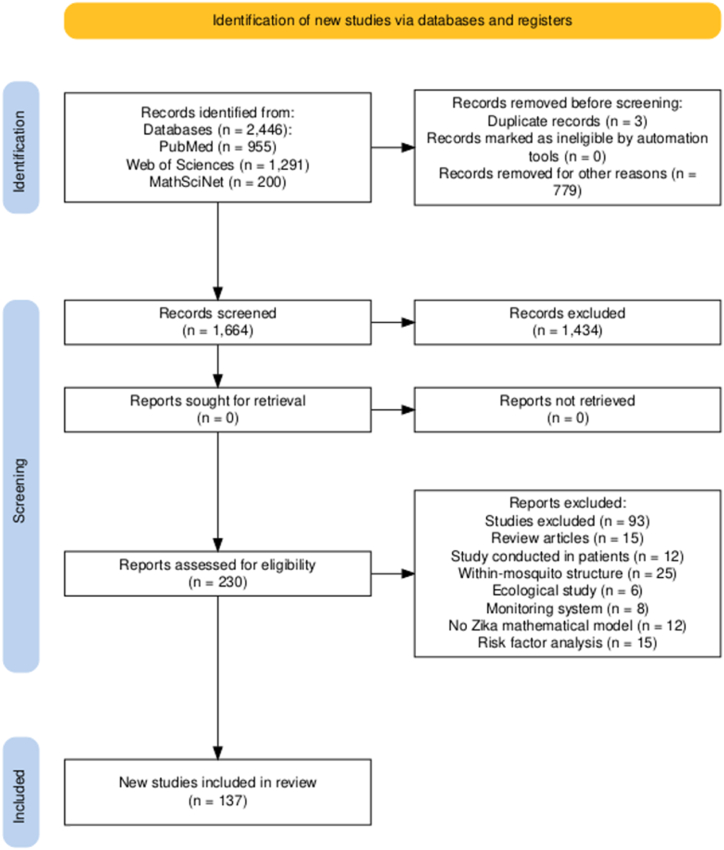
寨卡病毒(ZIKV)感染以及其他虫媒病毒的同步传播对公共卫生构成巨大挑战,提醒人们利用数学模型作为解释其复杂动态和与共传播病原体相互作用的关键工具。通过一项范围综述,我们旨在识别当前研究寨卡病毒动力学的数学模型,重点关注其与其他病原体的相互作用,并确定支持关注的潜在假设和缺陷,特别是关于寨卡病毒爆发的流行病学属性。遵循PRISMA-Sc指南,对PubMed、Web of Science和MathSciNet进行了系统搜索,从2446篇论文的初始库中提供了137项相关研究,显示了建模方法的多样性,主要集中在媒介-宿主区室模型上,并特别关注2015-2016年寨卡疫情期间哥伦比亚和巴西的流行病学景观。虽然迄今为止,建模研究在解释寨卡病毒传播动力学及其与登革热、基孔肯雅热和COVID-19等疾病的交叉方面非常重要,但未来的寨卡病毒模型应优先考虑强大的数据整合和针对不同数据集的严格验证,以提高流行病预测的准确性和可靠性。此外,模型可以从包含人类行为、环境因素和随机参数的适应性框架中受益,重点是开放获取工具,以促进透明度和研究合作。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exploring Zika's dynamics: A scoping review journey from epidemic to equations through mathematical modelling
Zika virus (ZIKV) infection, along with the concurrent circulation of other arboviruses, presents a great public health challenge, reminding the utilization of mathematical modelling as a crucial tool for explaining its intricate dynamics and interactions with co-circulating pathogens. Through a scoping review, we aimed to discern current mathematical models investigating ZIKV dynamics, focusing on its interplay with other pathogens, and to identify underlying assumptions and deficiencies supporting attention, particularly regarding the epidemiological attributes characterizing Zika outbreaks. Following the PRISMA-Sc guidelines, a systematic search across PubMed, Web of Science, and MathSciNet provided 137 pertinent studies from an initial pool of 2446 papers, showing a diversity of modelling approaches, predominantly centered on vector-host compartmental models, with a notable concentration on the epidemiological landscapes of Colombia and Brazil during the 2015–2016 Zika epidemic. While modelling studies have been important in explaining Zika transmission dynamics and their intersections with diseases such as Dengue, Chikungunya, and COVID-19 so far, future Zika models should prioritize robust data integration and rigorous validation against diverse datasets to improve the accuracy and reliability of epidemic prediction. In addition, models could benefit from adaptable frameworks incorporating human behavior, environmental factors, and stochastic parameters, with an emphasis on open-access tools to foster transparency and research collaboration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Infectious Disease Modelling
Mathematics-Applied Mathematics
CiteScore
17.00
自引率
3.40%
发文量
73
审稿时长
17 weeks
期刊介绍:
Infectious Disease Modelling is an open access journal that undergoes peer-review. Its main objective is to facilitate research that combines mathematical modelling, retrieval and analysis of infection disease data, and public health decision support. The journal actively encourages original research that improves this interface, as well as review articles that highlight innovative methodologies relevant to data collection, informatics, and policy making in the field of public health.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


