大麻二酚在lenox - gastaut综合征和Dravet综合征患者中的使用:一项回顾性图表回顾研究。
IF 1.5
Q3 CLINICAL NEUROLOGY
引用次数: 0
摘要
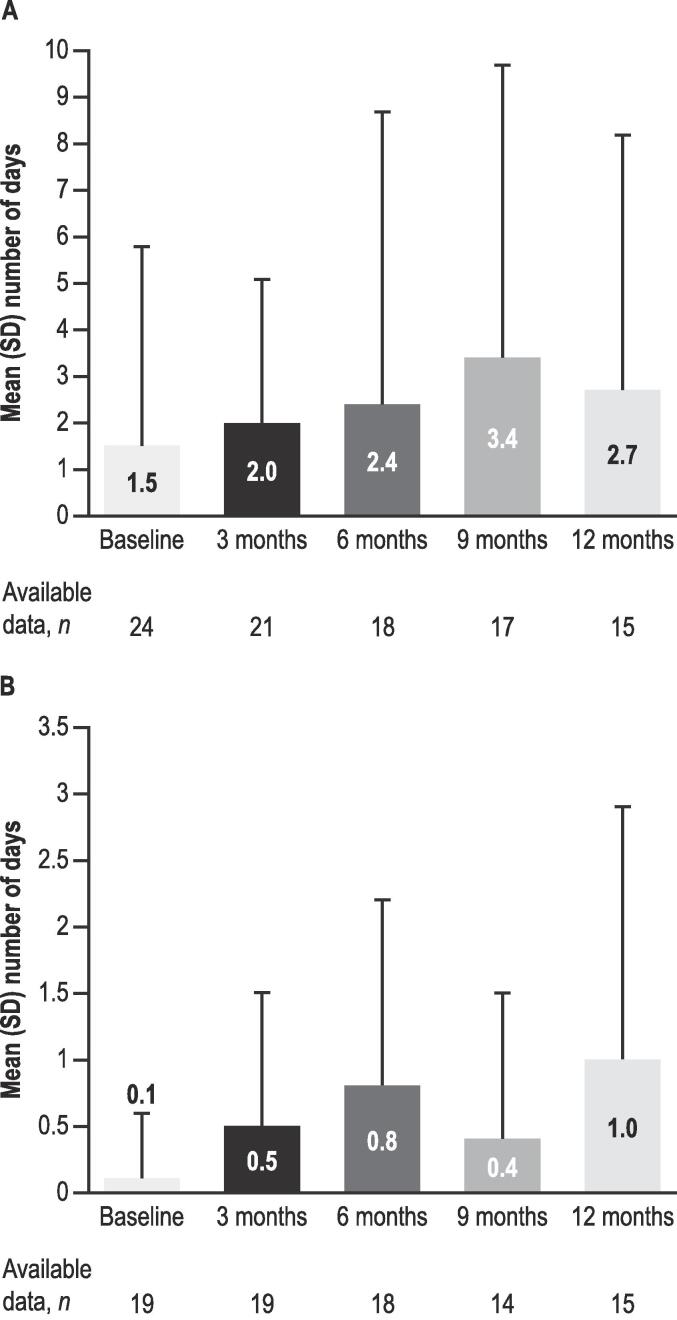
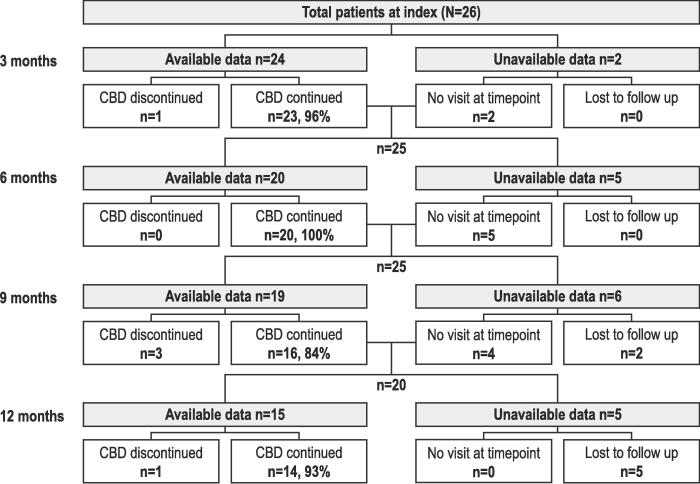
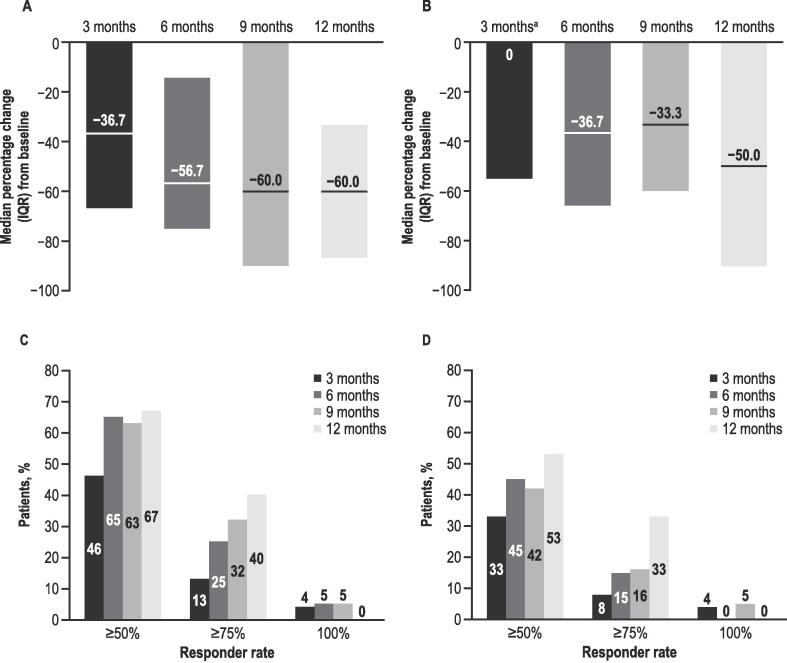
目的:评估英国早期用药计划中2-17岁lenox - gastaut综合征(LGS)或Dravet综合征(DS)患者接受植物源性高纯度大麻二酚(CBD)治疗的临床结果;Epidyolex®;100mg /mL口服液)。方法:回顾性图表回顾从基线(CBD治疗开始前1个月)到治疗12个月、CBD停药、死亡或失去随访收集的数据。结果:在基线时,所有26例患者入组(LGS, n = 17;DS, n = 9;男性,73%;平均年龄:11.8岁(3.0-17.0岁);92%的患者服用≥1种抗癫痫药物。6个月时CBD中位(IQR)剂量(6 M;n = 12)为6.0 (2.7)mg/kg/天,12个月(12 M;N = 9) 7.3 (2.1) mg/kg/天。运动癫痫发作的中位数(IQR)与基线的变化百分比在6 M (n = 20)时为- 56.7%(60.7),在12 M (n = 15)时为- 60.0%(53.3)。运动癫痫发作减少≥50%和≥75%的患者在6 M时分别为13/20(65%)和5/20(25%),在12 M时分别为10/15(67%)和6/15(40%)。平均(SD)无运动癫痫发作天数/月在基线时为1.5(4.3)天(n = 24,缺失数据n = 2), 6 M时为2.4(6.3)天(n = 18), 12 M时为2.7(5.5)天(n = 15)。在12点时,随访数据中患者的CBD保留率为14/19(74%),而7/26(27%)丢失。在6 M和12 M时,报告≥1个特殊不良事件(最常见:胃肠道)的患者人数分别为14/20(70%)和8/15(53%)。结论:结果表明运动癫痫发作减少,安全性与先前的研究一致。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The use of cannabidiol in patients with Lennox-Gastaut syndrome and Dravet syndrome in the UK Early Access Program: A retrospective chart review study
Purpose
To evaluate clinical outcomes from the UK Early Access Program in patients aged 2–17 years with Lennox-Gastaut syndrome (LGS) or Dravet syndrome (DS) treated with plant-derived highly purified cannabidiol (CBD; Epidyolex®; 100 mg/mL oral solution).
Methods
Retrospective chart review of data collected from baseline (1 month before CBD treatment initiation) until 12 months’ treatment, CBD discontinuation, death, or loss to follow up.
Results
At baseline, all 26 patients enrolled (LGS, n = 17; DS, n = 9; male, 73 %; mean [range] age, 11.8 [3.0–17.0] years) experienced motor seizures; 92 % were taking ≥ 1 antiseizure medication. Median (IQR) CBD dosage at 6 months (6 M; n = 12) was 6.0 (2.7) mg/kg/day, and 12 months (12 M; n = 9) 7.3 (2.1) mg/kg/day. Median (IQR) percentage change from baseline for motor seizures was − 56.7 % (60.7) at 6 M (n = 20), and − 60.0 % (53.3) at 12 M (n = 15). Patients experiencing ≥ 50 % and ≥ 75 % reduction in motor seizures were 13/20 (65 %) and 5/20 (25 %) at 6 M, respectively, and 10/15 (67 %) and 6/15 (40 %) at 12 M, respectively. Mean (SD) motor seizure-free days/month were 1.5 (4.3) at baseline (n = 24, missing data n = 2), 2.4 (6.3) at 6 M (n = 18), and 2.7 (5.5) at 12 M (n = 15). At 12 M, CBD retention for patients with follow-up data was 14/19 (74 %), whilst 7/26 (27 %) were lost to follow up. The number of patients reporting ≥ 1 adverse event of special interest (most common: gastrointestinal) was 14/20 (70 %) and 8/15 (53 %) at 6 M and 12 M, respectively.
Conclusion
Results demonstrate a reduction in motor seizures and a safety profile consistent with previous studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Epilepsy and Behavior Reports
Medicine-Neurology (clinical)
CiteScore
2.70
自引率
13.30%
发文量
54
审稿时长
50 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


