细胞色素P450酶产物释放与溶剂化关系的研究
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
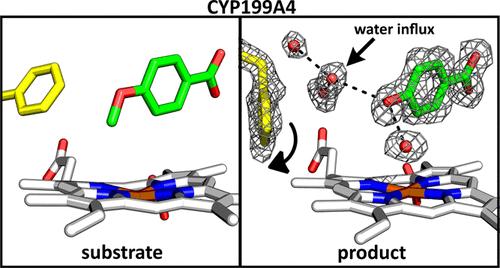
细胞色素P450酶(CYPs)是催化氧化反应的血红素硫酸酯单加氧酶。底物和抑制剂的结合可以使用一套标准技术进行评估,但对产物如何在活性位点结合知之甚少。虽然底物结合和产物从活性位点去除通常不是决定速率的步骤,但它们是多步催化循环和酶选择性的重要组成部分。来自palustris Rhodopseudomonas HaA2的细菌P450酶CYP199A4可催化对取代苯甲酸的高选择性氧化反应,如4-甲氧基苯甲酸氧化o -去甲基化为4-羟基苯甲酸和4-甲基苯甲酸羟基化为4-(羟甲基)苯甲酸。在这里,我们使用紫外可见吸收光谱、生化分析、x射线晶体学和分子动力学(MD)模拟来研究这些反应产物与这种酶的结合。实验结果表明,第6种水配体在加入任何一种产物配体时都不会发生位移,并且它们的结合强度低于它们各自的底物。结构变化包括活性位点水分子数量的增加,以及观察到几个疏水氨基酸残基位置的变化。将这些实验结果与模拟CYP199A4结合的4-甲氧基苯甲酸底物和4-羟基苯甲酸产物的计算结果进行了比较。本研究结合实验和理论分析,详细阐述了该酶如何与底物紧密结合并有效释放产物,促进高效催化,溶剂分子在产物释放过程中发挥重要作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Investigating the Correlation between Product Release and Solvation in Cytochrome P450 Enzymes
Cytochrome P450 enzymes (CYPs) are heme-thiolate monooxygenases that catalyze oxidation reactions. The binding of substrates and inhibitors can be assessed using a set of standard techniques, but less is known about how the products bind within the active site. Although substrate binding and product removal from the active site are generally not rate-determining steps, they are important components of the multistep catalytic cycle and the selectivity of the enzyme. The bacterial P450 enzyme CYP199A4, from Rhodopseudomonas palustris HaA2, catalyzes highly selective oxidation reactions on para-substituted benzoic acids such as the oxidative O-demethylation of 4-methoxybenzoic acid to 4-hydroxybenzoic acid and the hydroxylation of 4-methylbenzoic acid to 4-(hydroxymethyl)benzoic acid. Here, we examine the binding of the products of these reactions to this enzyme using UV–visible absorbance spectroscopy, biochemical assays, X-ray crystallography, and molecular dynamics (MD) simulations. Experimental results show that the sixth aqua ligand is not displaced on addition of either product ligand and they bind less tightly than their respective substrates. Structural changes included an increase in the number of active site water molecules present, and changes in the position of several hydrophobic amino acid residues were observed. These experimental findings were compared with computational studies simulating both the 4-methoxybenzoic acid substrate and 4-hydroxybenzoic acid product bound to CYP199A4. Combining experimental and theoretical analyses, this study provides a detailed molecular rationale on how this enzyme can bind its substrates tightly yet effectively release the products, facilitating efficient catalysis with solvent molecules playing an important role in the process of product release.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


