mo掺杂诱导富氧空位Bi4O5Br2纳米片带结构调控以改善可见光催化
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
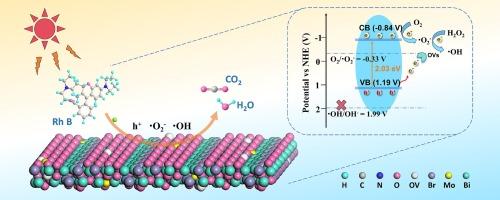
光催化是一种高效、低能耗、环保的污染物处理技术。由于其独特的电子结构,Bi4O5Br2在BixOyBrz材料中成为一种很有前途的光催化剂,但它受到光生载流子重组的阻碍。基于Bi-O键的不稳定性,本工作利用Mo掺杂和氧空位(OVs)的协同效应增强了改性Bi4O5Br2的带隙结构,改善了光吸收,促进了电荷分离。其中,3 % Mo-Bi4O5Br2表现出最好的光催化性能,光照40 min后对罗丹明B (Rh B)的降解率达到98.40 %,比未掺杂的Bi4O5Br2的降解率高3.28倍。此外,该催化剂在10个循环后,在各种条件下,包括共存离子、溶液pH和其他有机染料的存在,都能保持优异的催化活性。通过绿豆发芽试验,探讨了降解产物的生物相容性,并对Rh B的降解机理进行了探讨。本研究提高了人们对掺杂与OVs协同作用对有机污染物的有效降解的认识,并为提高催化剂的光催化活性提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mo-doping induced band structures regulation in oxygen vacancies-rich Bi4O5Br2 nanosheets for improved visible-light photocatalysis
Photocatalysis is an effective, low-energy and environmentally friendly technology for the treatment of pollutants. Bi4O5Br2, distinguished by its unique electronic structure, emerges as a promising photocatalyst among BixOyBrz materials, yet it is hindered by significant photogenerated carrier recombination. Based on the instability of the Bi–O bond, this work utilizes the synergistic effect of Mo doping and oxygen vacancies (OVs) to enhance the bandgap structure of the modified Bi4O5Br2, improving light absorption and facilitating charge separation. Among the samples, 3 % Mo-Bi4O5Br2 exhibits the best photocatalytic performance, achieving a degradation rate of 98.40 % for Rhodamine B (Rh B) after 40 min of light exposure, with a degradation rate constant 3.28 times higher than that of undoped Bi4O5Br2. Moreover, this catalyst maintains excellent catalytic activity after ten cycles and under various conditions, including the presence of coexisting ions, solution pH, and other organic dyes. The biocompatibility of the degradation products was explored using a mung bean sprouting experiment, and the degradation mechanism of Rh B was also discussed. This work enhances understanding of the effective degradation of organic pollutants through the synergistic effect of doping and OVs, and offers new insights into improving the photocatalytic activity of catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


