双核镍催化乙烯与短链烯醇单体共聚
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
乙烯与乙烯极性单体,特别是短链烯醇的配位共聚,为在温和条件下控制合成重要的羟基功能化聚乙烯提供了一种有吸引力的方法。然而,由于螯合配位和β-O消除等问题,短链烯醇共聚的报道有限。本文报道了与烯丙基- oh、3-丁烯-1-醇、4-戊烯-1-醇和9-十二烯-1-醇等烯醇单体共聚乙烯的双核Ni配合物的合成和表征。这些配合物经Et2AlCl活化后,在乙烯/3-丁烯-1-醇共聚中获得了显著的活性(高达592 kg (mol cat h atm) -1),共聚物掺入量为1.7 mol%,分子量为64.2 kg mol -1)。活性和共聚单体含量受Et2AlCl负载、反应温度和烯醇单体长度的影响,9-十二-1-醇等烯醇越长,活性越高,单体掺入量越大,分子量也越大。乙烯/烯丙基- oh共聚反应的活性可达169 kg (mol cat h atm)−1,分子量降低(Mn = 17.2 kg mol−1)。微观结构分析显示,在所有情况下,链内和链端极性单体结合明显。值得注意的是,乙烯/烯丙基- oh共聚物表现出独特的烯烃端基和可分配给Friedel-Crafts反应的微观结构,这可能是由于O原子和活性Ni中心之间的短链长度导致的替代链终止途径。相比之下,乙烯/烯丙基oac共聚物仅显示烯烃基团,表明β-OAc消除机制。这一过程降低了催化剂的活性和分子量,表明催化剂因快速断链而中毒。本文章由计算机程序翻译,如有差异,请以英文原文为准。

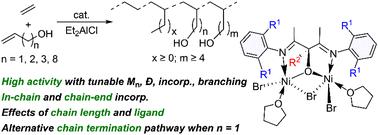
Binuclear Ni catalyzed ethylene copolymerization with short chain alkenol monomers†
Ethylene coordination copolymerization with vinyl polar monomers, particularly short chain alkenols, offers an attractive method for controlled synthesis of important hydroxy-functionalized polyethylenes under mild conditions. However, reports on short-chain alkenol copolymerization are limited due to issues like chelating coordination and β-O elimination. Here, we report the synthesis and characterization of binuclear Ni complexes for ethylene copolymerization with various alkenol monomers such as allyl-OH, 3-buten-1-ol, 4-penten-1-ol and 9-decen-1-ol. These complexes, upon activation with Et2AlCl, achieved notable activity (as high as 592 kg (mol cat h atm)−1) in ethylene/3-buten-1-ol copolymerization, producing copolymers with 1.7 mol% comonomer incorporation and a high molecular weight (Mn = 64.2 kg mol−1). The activity and comonomer content were influenced by Et2AlCl loading, reaction temperature, and alkenol monomer length, with longer alkenols such as 9-decen-1-ol yielding higher activity, comonomer incorporation and molecular weight. Activities up to 169 kg (mol cat h atm)−1 were also achieved in ethylene/allyl-OH copolymerization with reduced molecular weight (Mn = 17.2 kg mol−1). Microstructural analysis revealed predominant in-chain and chain-end polar monomer incorporation in all cases. Notably, ethylene/allyl-OH copolymers exhibited unique olefinic end groups and microstructures assignable to Friedel–Crafts reactions, which is likely due to an alternative chain termination pathway associated with the short chain length between the O atom and the active Ni center. For comparison, ethylene/allyl-OAc copolymers showed exclusively olefinic groups, indicating a β-OAc elimination mechanism. This process resulted in lower activity and molecular weight, suggesting catalyst poisoning from rapid chain termination.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


