Septin组装促进膜的脂质组织
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
细胞骨架介导的膜区隔化对支持细胞功能至关重要,从信号传导到细胞分裂、迁移或吞噬。septin是直接与膜相互作用的细胞骨架蛋白,作为支架将蛋白质招募到细胞位置并作为结构扩散屏障。septin如何与膜的脂质组织相互作用和重塑尚不清楚。在这里,我们结合了最小重组系统和酵母细胞成像来研究septin介导的膜组织。我们的研究结果表明,在低浓度下,膜弥散性隔素自组装成亚微米级的斑块,与隔素环共存于分裂位点。我们发现斑块是由短的septin丝组成的,并且能够调节膜的脂质组织。此外,我们发现Cdc11的多基结构域影响了septin的膜组织和曲率传感特性。总的来说,我们的工作提供了对septin支持与膜密切相关的细胞功能的分子机制的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
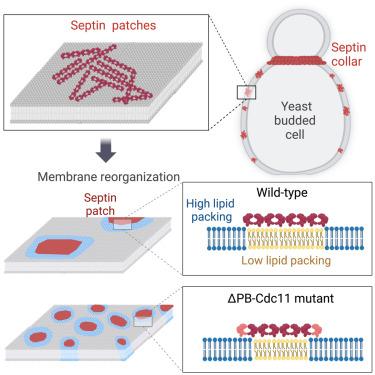
Septin assemblies promote the lipid organization of membranes
Cytoskeletal-mediated membrane compartmentalization is essential to support cellular functions, from signaling to cell division, migration, or phagocytosis. Septins are cytoskeletal proteins that directly interact with membranes, acting as scaffolds to recruit proteins to cellular locations and as structural diffusion barriers. How septins interact with and remodel the lipid organization of membranes is unclear. Here, we combined minimal reconstituted systems and yeast cell imaging to study septin-mediated membrane organization. Our results show that at low concentrations membrane-diffusive septins self-assemble into sub-micrometric patches that co-exist with the septin collar at the division site. We found that patches are made of short septin filaments and that are able to modulate the lipid organization of membranes. Furthermore, we show that the polybasic domain of Cdc11 influences the membrane-organizing and curvature-sensing properties of septins. Collectively, our work provides understanding of the molecular mechanisms by which septins can support cellular functions intimately linked to membranes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


