微生物来源的琥珀酸盐通过赖氨酸琥珀酰化促进肠出血性大肠杆菌的毒力
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
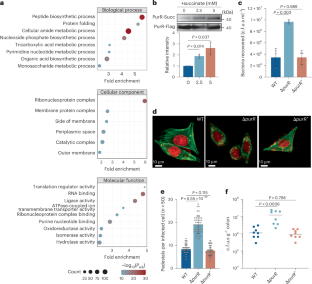
琥珀酸盐上调肠出血性大肠杆菌(EHEC)的毒力。赖氨酸琥珀酰化是一种翻译后修饰,在真核生物中调节细胞功能,但在细菌中较少表征。我们假设赖氨酸琥珀酰化调节肠出血性大肠杆菌的毒力。在这里,我们使用基于silac的蛋白质组学并对肠出血性大肠杆菌琥珀酰化酶进行了表征,结果表明转录因子PurR在K24和K55位点琥珀酰化。PurR的琥珀酰化抑制了其直接结合DNA的能力,抑制了主要毒力因子3型分泌系统(T3SS)的表达,从而增加了T3SS的表达。purR或K24E或K55E突变的缺失,增加了肠出血性大肠杆菌对细胞的粘附和幼兔的定植。通过使用链霉素处理的小鼠来消耗琥珀酸,或者用产琥珀酸的copvotella定植增加琥珀酸水平,我们发现微生物来源的琥珀酸增加了PurR的琥珀化,从而促进了鼠柠檬酸杆菌(一种肠出血性大肠杆菌模型)在小鼠中的毒力。最后,我们确定了CitC是PurR修饰所需的琥珀基转移酶。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Microbiota-derived succinate promotes enterohaemorrhagic Escherichia coli virulence via lysine succinylation
Succinate upregulates enterohaemorrhagic Escherichia coli (EHEC) virulence. Lysine succinylation, a post-translational modification, regulates cellular function in eukaryotes but is less characterized in bacteria. We hypothesized that lysine succinylation regulates EHEC virulence. Here we used SILAC-based proteomics and characterized the EHEC succinylome to show that the transcription factor, PurR, is succinylated at K24 and K55. Succinylation of PurR inhibited its ability to directly bind DNA and repress expression of a major virulence factor, the Type 3 Secretion System (T3SS), thus increasing T3SS expression. Deletion of purR, or K24E or K55E mutation, increased EHEC adherence to cells and colonization of infant rabbits. Using mice treated with streptomycin to deplete succinate, or colonized with succinate-producing Prevotella copri to increase succinate levels, we showed that microbiota-derived succinate increased succinylation of PurR to promote virulence of Citrobacter rodentium, a model for EHEC, in mice. Lastly, we identified CitC as the succinyltransferase required for PurR modification. Gut microbiota-derived succinate promotes CitC succinyltransferase-dependent succinylation of PurR to alleviate T3SS repression and promote virulence in enterohaemorrhagic Escherichia coli and Citrobacter rodentium.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


