通过TLR9的损伤感知调节流感感染期间的炎症和抗病毒反应。
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
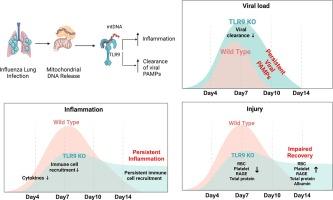
宿主反应旨在消除感染病原体,以及病原体本身,可导致组织损伤。组织损伤导致包括线粒体DNA在内的无数细胞成分的释放,宿主通过模式识别受体感知这些成分。宿主对组织损伤的感知如何形成抗病原体反应仍然知之甚少。在这项研究中,我们使用了toll样受体-9 (TLR9)缺乏的小鼠,TLR9与未甲基化的CpG DNA序列结合,如细菌和线粒体DNA中的CpG DNA序列。为了避免TLR9直接感知病原体,我们利用缺乏TLR9配体的流感病毒,来确定TLR9的损伤感知如何促进抗流感免疫。我们的数据表明,tlr9介导的组织损伤感知在早期感染期间促进了炎症反应,由上皮细胞和髓细胞驱动。随着炎症反应的减弱,TLR9的缺失导致病毒清除受损,表现为骨髓细胞(包括单核细胞和巨噬细胞)中流感成分的增加和延长,使其高度炎症。在流感感染的TLR9-/-小鼠中,受感染骨髓细胞驱动的持续炎症导致持久性肺损伤和恢复受损。此外,我们发现流感患者血浆样本中TLR9激活升高,并与住院患者疾病严重程度相关,证明其临床相关性。总的来说,我们证明了通过TLR9损伤感知在促进抗流感免疫和炎症反应中的重要作用。作者总结:组织损伤是临床相关肺部感染的必然结果,但在感染过程中检测这种损伤的宿主机制尚不清楚。我们研究toll样受体9 (TLR9)在感知流感引起的组织损伤中的作用。由于流感缺乏TLR9配体,我们假设TLR9信号是由组织损伤分子如线粒体DNA (mtDNA)驱动的。我们的数据表明,TLR9减少了早期炎性肺损伤,但损害了病毒清除,导致广泛的免疫细胞感染、持续炎症和延迟恢复。髓细胞特异性TLR9缺失可改善晚期炎症反应。在人类中,与健康对照组相比,流感感染者血浆中TLR9活性和mtDNA水平升高,TLR9激活电位升高与需要进入ICU的严重疾病相关。这些发现表明,tlr9介导的损伤感知触发炎症组织损伤和病毒清除。这些数据表明,TLR9活性可以作为限制流感诱导的组织损伤的重要生物标志物和治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Damage sensing through TLR9 regulates inflammatory and antiviral responses during influenza infection
Host response aimed at eliminating the infecting pathogen, as well as the pathogen itself, can cause tissue injury. Tissue injury leads to the release of a myriad of cellular components including mitochondrial DNA (mtDNA), which the host senses through pattern recognition receptors. How the sensing of tissue injury by the host shapes the anti-pathogen response remains poorly understood. In this study, we utilized mice that are deficient in toll-like receptor-9 (TLR9), which binds to unmethylated CpG DNA sequences such as those present in bacterial and mtDNA. To avoid direct pathogen sensing by TLR9, we utilized the influenza virus, which lacks ligands for TLR9, to determine how damage sensing by TLR9 contributes to anti-influenza immunity. Our data showed that TLR9-mediated sensing of tissue damage promoted an inflammatory response during early infection, driven by epithelial and myeloid cells. Along with the diminished inflammatory response, the absence of TLR9 led to impaired viral clearance manifested as higher and prolonged influenza components in myeloid cells, including monocytes and macrophages, rendering them highly inflammatory. The persistent inflammation driven by infected myeloid cells led to persistent lung injury and impaired recovery in influenza-infected TLR9-/- mice. Further, we found elevated TLR9 ligands in the plasma samples of patients with influenza infection and its association with the disease severity in hospitalized patients, demonstrating its clinical relevance. Overall, we demonstrated an essential role of damage sensing through TLR9 in promoting anti-influenza immunity and inflammatory response.
Author Summary
Tissue damage is an inevitable outcome of clinically relevant lung infections, but the host mechanisms for detecting such damage during infection are not well understood. We investigated the role of Toll-like receptor 9 (TLR9) in sensing tissue damage caused by influenza. Since influenza lacks TLR9 ligands, we hypothesized that TLR9 signaling is driven by tissue damage molecules like mitochondrial DNA (mtDNA). Our data revealed that TLR9 deficiency reduces early inflammatory lung injury but impairs viral clearance, resulting in extensive infection of immune cells, persistent inflammation, and delayed recovery. Myeloid-specific TLR9 deletion ameliorated late-stage inflammatory responses. In humans, influenza-infected individuals exhibited elevated TLR9 activity and mtDNA levels in plasma compared to healthy controls, with higher TLR9 activation potential correlating with severe disease requiring ICU admission. These findings suggest that TLR9-mediated damage sensing triggers both inflammatory tissue injury and viral clearance. These data indicate that TLR9 activity can serve as a crucial biomarker and therapeutic target to limit influenza-induced tissue injury.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Mucosal Immunology
医学-免疫学
CiteScore
16.60
自引率
3.80%
发文量
100
审稿时长
12 days
期刊介绍:
Mucosal Immunology, the official publication of the Society of Mucosal Immunology (SMI), serves as a forum for both basic and clinical scientists to discuss immunity and inflammation involving mucosal tissues. It covers gastrointestinal, pulmonary, nasopharyngeal, oral, ocular, and genitourinary immunology through original research articles, scholarly reviews, commentaries, editorials, and letters. The journal gives equal consideration to basic, translational, and clinical studies and also serves as a primary communication channel for the SMI governing board and its members, featuring society news, meeting announcements, policy discussions, and job/training opportunities advertisements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


