有机光电氧化还原驱动的三组分合成β-三氟甲基β-氨基酮†。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-02-14
Epub Date: 2025-01-30
DOI:10.1021/acs.joc.4c03142
引用次数: 0
摘要
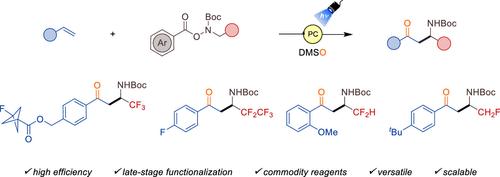
在这项工作中,我们提出了一种光氧化还原三组分反应,可以从n -三氟乙基羟胺衍生物、苯乙烯和二甲基亚砜合成药用相关的β-三氟甲基β-氨基酮。值得注意的是,氟甲基、二氟甲基和五氟乙基类似物也可在相同的反应条件下获得。机理研究,包括自由基捕获实验、循环伏安法、Stern-Volmer猝灭研究和同位素标记实验,支持光诱导自由基/极性交叉和kornblum型氧化机制。最后,通过显著的衍生化反应证明了所获得的有机骨架的适用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Organophotoredox-Driven Three-Component Synthesis of β-Trifluoromethyl β-Amino Ketones†.
In this work, we present a photoredox three-component reaction that enables the synthesis of medicinally relevant β-trifluoromethyl β-amino ketones from a N-trifluoroethylhydroxylamine derivative, styrenes and DMSO. Remarkably, fluoromethyl, difluoromethyl and pentafluoroethyl analogues are also accessed using the same reaction conditions. The mechanistic investigations, including radical trapping experiments, cyclic voltammetry, Stern-Volmer quenching studies and isotope labelling experiments support the photoinduced radical/polar crossover and Kornblum-type oxidation mechanisms. Finally, the applicability of the accessed organic skeletons is showcased by notable derivatization reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


