高效电化学制冷用热电电解质的溶剂化熵工程
IF 38.6
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
新兴的热电系统不仅可以将热转化为电,而且还可以实现电化学制冷。然而,由于缺乏合理的设计原则,它们的基本电解质在高冷却性能方面面临挑战。开发具有高温系数和低热容量的热电偶电解质是实现高效电化学制冷的关键。本文报道了一种基于二元溶剂和阴离子协同工程的铁基电解质设计策略,该策略重新排列了Fe2+/3+离子的溶剂化壳,从而实现了3.73 mV K−1的高温系数,同时降低了热容量。综合分析表明,Fe2+/3+-ClO4−的弱相互作用,以及Fe2+和腈溶剂之间的选择性结合,充分扩大了电化学制冷的熵变。结果表明,优化后的电解质可以将冷却功率提高约70%,并且仅以0.11 W cm−2的输入就可以直接冷却约1.42 K的电解质,这表明了实际电化学制冷的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
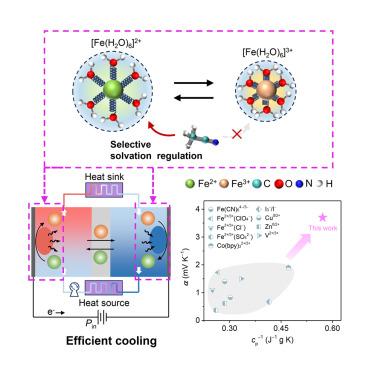

Solvation entropy engineering of thermogalvanic electrolytes for efficient electrochemical refrigeration
Emerging thermogalvanic systems can not only convert heat into electricity but also enable electrochemical refrigeration. However, their fundamental electrolytes meet challenges toward high cooling performance due to the absence of rational design principles. Developing thermogalvanic electrolytes with high-temperature coefficients and low heat capacity is the key to efficient electrochemical refrigeration. Here, we report an iron-based electrolyte design strategy by synergistic binary solvent and anion engineering, which rearranges the solvation shell of Fe2+/3+ ions to achieve a high-temperature coefficient of 3.73 mV K−1 with decreased heat capacity. The comprehensive analyses reveal that the weak Fe2+/3+-ClO4− interactions, accompanied by selective association between Fe2+ and nitrile solvents, fully enlarge the entropy change available for electrochemical refrigeration. As a result, the optimized electrolyte could potentially reach ∼70% improvement of cooling power, and a direct cooling of electrolyte ∼1.42 K was demonstrated with only 0.11 W cm−2 input, showing promise for practical electrochemical refrigeration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Joule
Energy-General Energy
CiteScore
53.10
自引率
2.00%
发文量
198
期刊介绍:
Joule is a sister journal to Cell that focuses on research, analysis, and ideas related to sustainable energy. It aims to address the global challenge of the need for more sustainable energy solutions. Joule is a forward-looking journal that bridges disciplines and scales of energy research. It connects researchers and analysts working on scientific, technical, economic, policy, and social challenges related to sustainable energy. The journal covers a wide range of energy research, from fundamental laboratory studies on energy conversion and storage to global-level analysis. Joule aims to highlight and amplify the implications, challenges, and opportunities of novel energy research for different groups in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


