使用基于方酰胺的离子对受体在三相结的不混溶液体之间转移高亲水性离子
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
尽管许多氧化还原探针已用于三相连接装置中阴离子转移的研究,但没有已知的分子能够转移高度亲水的阴离子。近年来,由冠醚阳离子结合位点和方酰胺阴离子结合域组成的离子对受体的研究不断涌现,这些受体具有提取极亲水性硫酸盐的能力。在本文中,我们表明,我们可以诱导转移硫酸盐和氟阴离子在三相电极设置使用氧化的方酰胺基化合物在有机相。我们评估了该化合物对硫酸盐和氟离子的转移及其对其他阴离子的选择性。我们对常规电化学电池中的离子转移进行了测量,在玻碳电极表面沉积了一个小的有机液滴。这些测量证明了离子转移伏安法在有机化合物表征和传感中的应用潜力本文章由计算机程序翻译,如有差异,请以英文原文为准。
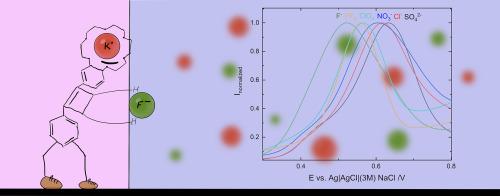
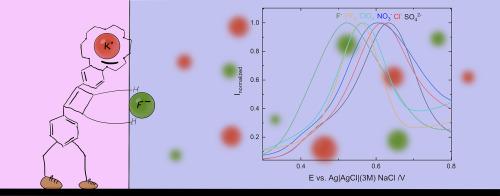
Transfer of highly hydrophilic ions between immiscible liquids at the three-phase junction using a squaramide-based ion pair receptor
Although many redox probes have been used for anion transfer studies in three-phase junction setups, no known molecule would enable the transfer of highly hydrophilic anions. In recent years, research has been emerging on ion pair receptors consisting of a crown ether cation binding site and a squaramide anion binding domain, which offer the ability to extract extremely hydrophilic sulphate salts. In this paper, we show that we can induce the transfer of sulphate and fluoride anions at a three-phase electrode setup using the oxidation of a squaramide-based compound in the organic phase. We evaluate the compound for the transfer of sulphate and fluoride ions and its selectivity towards other anions. We performed measurements of the transfer of ions in regular electrochemical cells, with a small organic drop deposited onto the surface of a glassy carbon electrode. These measurements demonstrate the potential of applying ion-transfer voltammetry in the characterization of organic compounds and sensing
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


