肠道内神经元与ILC2s的双向交流抑制宿主对蠕虫感染的防御
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
新的研究表明,神经递质和神经肽在调节抗蠕虫免疫反应中起关键作用,暗示肠道内神经元(iENs)在协调肠道免疫中的潜力。iENs是否以及如何在感染期间被激活,以及潜在的神经免疫相互作用仍然不清楚。在这里,我们发现蠕虫感染激活了iENs的一个子集。iENs的单核RNA测序(snRNA-seq)揭示了白细胞介素(IL)-13R+内在初级传入神经元(IPANs)转录谱的改变,包括神经肽β-降钙素基因相关肽(CGRP)的上调。利用遗传小鼠模型和工程病毒工具,我们证明了2组先天淋巴样细胞(ILC2)衍生的IL-13需要通过IL-13R激活iENs,导致iEN产生β-CGRP,随后抑制ILC2反应和抗蠕虫免疫。总之,这些结果揭示了肠道中iENs和ILC2s亚群之间以前未被识别的双向神经免疫串扰,这影响了病原体的清除。本文章由计算机程序翻译,如有差异,请以英文原文为准。
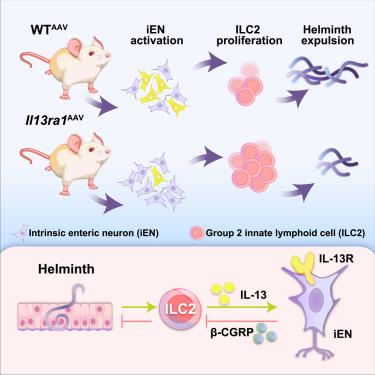
Bi-directional communication between intrinsic enteric neurons and ILC2s inhibits host defense against helminth infection
Emerging studies reveal that neurotransmitters and neuropeptides play critical roles in regulating anti-helminth immune responses, hinting at the potential of intrinsic enteric neurons (iENs) in orchestrating intestinal immunity. Whether and how iENs are activated during infection and the potential neuroimmune interactions involved remain poorly defined. Here, we found that helminth infection activated a subset of iENs. Single-nucleus RNA sequencing (snRNA-seq) of iENs revealed alterations in the transcriptional profile of interleukin (IL)-13R+ intrinsic primary afferent neurons (IPANs), including the upregulation of the neuropeptide β-calcitonin gene-related peptide (CGRP). Using genetic mouse models and engineered viral tools, we demonstrated that group 2 innate lymphoid cell (ILC2)-derived IL-13 was required to activate iENs via the IL-13R, leading to iEN production of β-CGRP, which subsequently inhibited ILC2 responses and anti-helminth immunity. Together, these results reveal a previously unrecognized bi-directional neuroimmune crosstalk in the intestine between a subset of iENs and ILC2s, which influences pathogen clearance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


