鸟嘌呤辅助低pt集成Mo2C/C析氢反应
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
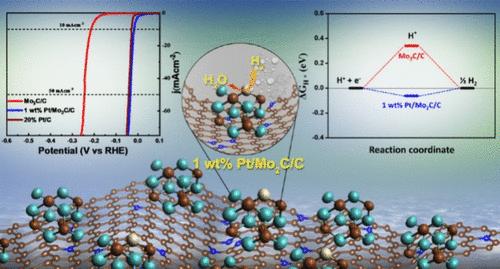
由于现有Pt电催化剂价格昂贵,阻碍了大规模水电解制氢的发展。通过电化学水分解制氢是可持续发展社会必不可少的可再生能源,它创造了一种独特的材料界面,显示出类似pt的性质,对析氢反应(HERs)具有长期稳定性。本文采用固体煅烧法和化学还原法制备了鸟嘌呤辅助易合成的1 wt % Pt/Mo2C/C层状材料。利用电感耦合等离子体发射光谱(ICP-OES)对发育良好的1 wt % Pt/Mo2C/C异质结构进行了分析,以了解Pt掺杂在Mo2C/C上的百分比。合成的1 wt % Pt/Mo2C/C异质结构具有比商用Pt/C更好的HER活性,过电位较小,为19 mV,电流密度为10 mA cm-2, Tafel斜率为28 mV/dec。1 wt % Pt/Mo2C/C催化剂在0.5 M H2SO4中表现出42 h的长期稳定性。n掺杂碳(C)纳米片的层状结构,在层内包裹了分散良好的Pt,显著提高了1wt % Pt/Mo2C/C的反应动力学。该设计在Mo2C、Pt和碳基体之间创造了协同效应,提高了催化性能。利用密度泛函理论(DFT)进行理论计算,确定了pt集成Mo2C/C上析氢的活性位点。与原始Mo2C/C材料(ΔGH* = 0.34 eV)相比,1 wt % Pt/Mo2C/C材料具有显著降低的ΔGH*值(- 0.06 eV),表明具有更高的催化活性。这种简单的方法提供了一种新的方法来制造明确定义的碳化物,并为可扩展HER的低铂催化剂的制造提供了新的思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Guanine-Assisted Contrived Low Pt-Integrated Mo2C/C for Hydrogen Evolution Reaction
Due to the high cost of the available Pt electrocatalysts, the large-scale water electrolysis production of hydrogen has been hindered. Hydrogen generation via electrochemical water splitting is a renewable energy essential to a sustainable society, creating a distinct material interface that shows Pt-like properties with long-term stability crucial to hydrogen evolution reactions (HERs). Here, we synthesized the guanine-assisted facile synthesis of 1 wt % Pt/Mo2C/C having a layered type morphology via solid state calcined process followed by chemical reduction. The well-developed 1 wt % Pt/Mo2C/C heterostructure is analyzed by inductively coupled plasma optical emission spectroscopy (ICP-OES) to understand the percentage of Pt doped on Mo2C/C. The as-synthesized 1 wt % Pt/Mo2C/C heterostructure exhibits a better HER activity than a commercial Pt/C with a small overpotential of 19 mV to reach a current density at 10 mA cm–2 with a Tafel slope of 28 mV/dec. The catalyst 1 wt % Pt/Mo2C/C shows a long-term stability of 42 h in 0.5 M H2SO4. The layered sheet structure with the N-doped carbon (C) nanosheet, encapsulating well-dispersed Pt within the layers, significantly enhances the reaction kinetics of the 1 wt % Pt/Mo2C/C. This design creates a synergistic effect among Mo2C, Pt, and the carbon matrix, improving catalytic performance. Theoretical calculations using the density functional theory (DFT) indicate the active sites for hydrogen evolution on Pt-integrated Mo2C/C. The 1 wt % Pt/Mo2C/C possessed a significantly reduced ΔGH* value (−0.06 eV) as compared to the pristine Mo2C/C material (ΔGH* = 0.34 eV), suggesting a higher catalytic activity. This simple method offers a fresh means to make clearly defined carbides and sheds light on creating low-Pt catalysts for a scalable HER.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


