烷氧基取代Lindqvist {MW5}和Keggin {MPW11}多金属氧酸盐的质子分解和缩合反应:比较实验和模拟研究
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
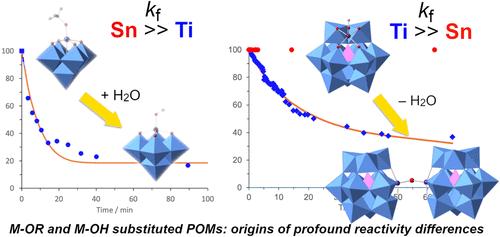
了解固体金属氧化物和相关的分子多金属氧酸盐(POMs)和金属烷氧化物表面的质子转移和迁移对于质子化或水的吸收/结合反应性的发展至关重要。在本研究中,醇氧基Ti-和sn -取代Lindqvist [(MeO)MW5O18]3 - (M = Ti, 1;M = Sn, 2)和Keggin [(MeO)MPW11O39]4 - (M = Ti, 3;本文详细研究了M = Sn, 4)型多金属氧酸盐(pom)生成羟基衍生物以及随后缩合成μ-氧化物的过程,为深入了解这些分子金属氧化物体系中的质子转移反应提供了依据。溶液核磁共振研究表明,反应不仅依赖于杂原子(Ti或Sn)的性质,而且还依赖于孔体(W5或PW11) POM的类型以及溶剂(MeCN或DMSO)。锡取代阴离子2和4比Ti类似物1和3更容易发生质子溶解,而{MW5}阴离子的反应通常比{MPW11}阴离子的反应快。随后缩合生成的羟基衍生物[(HO)MW5O18]3 - (M = Ti, 5;M = Sn, 6)和[(HO)MPW11O39]4 - (M = Ti, 7;M = Sn, 8)在5和7中明显更容易发生,并且在所有情况下,DMSO中的缩合都被抑制。通过核磁共振动力学实验分析,定量比较了平衡态和反应速率,而对这些反应和类似的{NbW5}反应的DFT计算提供了与实验观察一致的比较能量学和反应谱。这些结果增加了对金属醇盐水解/缩合和金属氧化物表面相关反应中质子迁移的基本理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Protonolysis and Condensation Reactions of Alkoxido-Substituted Lindqvist {MW5} and Keggin {MPW11} Polyoxometalates: Comparative Experimental and Modeling Studies
An understanding of proton transfer and migration at the surfaces of solid metal oxides and related molecular polyoxometalates (POMs) and metal alkoxides is crucial for the development of reactivity involving protonation or the absorption/binding of water. In this work, the hydrolysis of alkoxido Ti- and Sn-substituted Lindqvist [(MeO)MW5O18]3– (M = Ti, 1; M = Sn, 2) and Keggin [(MeO)MPW11O39]4– (M = Ti, 3; M = Sn, 4) type polyoxometalates (POMs) to hydroxido derivatives and subsequent condensation to μ-oxido species has been investigated in detail to provide insight into proton transfer reactions in these molecular metal oxide systems. Solution NMR studies revealed the dependence of reactions not only on the nature of the heteroatom (Ti or Sn) but also on the type of lacunary (W5 or PW11) POM and also on the solvent (MeCN or DMSO). Tin-substituted anions 2 and 4 were much more susceptible to protonolysis than the Ti analogues 1 and 3 while reactions of {MW5} anions were generally faster than those of the {MPW11} anions. Subsequent condensation of the resulting hydroxido derivatives [(HO)MW5O18]3– (M = Ti, 5; M = Sn, 6) and [(HO)MPW11O39]4– (M = Ti, 7; M = Sn, 8) was significantly more facile for 5 and 7 and, in all cases, condensation was inhibited in DMSO. Quantitative comparisons of equilibria and reaction rates were provided by analysis of NMR kinetic experiments, while DFT calculations on these and the analogous {NbW5} reactions provided comparative energetics and reaction profiles that are consistent with experimental observations. These results add to the fundamental understanding of proton migration in metal alkoxide hydrolysis/condensation and related reactions at metal oxide surfaces.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


