光动力疗法中候选光敏剂的构建:计算机辅助设计、计算和筛选
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
噻吩和吡咯单元在光响应材料中得到了广泛的应用,并大大推进了有机光伏(OPV)领域的发展。这一进展启发了我们对光动力治疗(PDT)用光敏剂(PS)的探索。目前,传统PS在临床应用中面临品种有限、适用范围狭窄等局限性。借鉴OPV的分子设计理念,我们的目标是在PDT中超越这些限制。考虑到候选分子的丰富程度,有效的筛选是至关重要的。理论计算和电子结构分析是精确实用的筛选方法。在本研究中,我们采用了OPV分子设计中成功应用的策略,重点关注供体-受体(D-A)和受体-供体-受体(A-D-A)结构。利用密度泛函理论(DFT)和时变密度泛函理论(TDDFT),系统地设计了有前途的有机碎片组合。这些片段包括以聚噻吩和聚吡咯为主的供体结构,与5个电子受体配对:茚(Ind)、二酮吡咯(DPP)、萘酰亚胺(Ni)、苯并噻唑(Btd)和二噻唑基二酮吡咯(Tbo)。通过细致的计算,我们获得了所有候选分子的电子结构和光谱性质,促进了高效的筛选过程。我们的研究结果强调,这些基于聚吡咯的框架与DPP、Ni和Btd的组合在PS应用中具有重要的前景。通过综合比较选择了约13%的候选分子,显著缩短了分子设计时间和实验成本。这种跨学科的方法有可能为更有针对性和成功的PS设计铺平道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
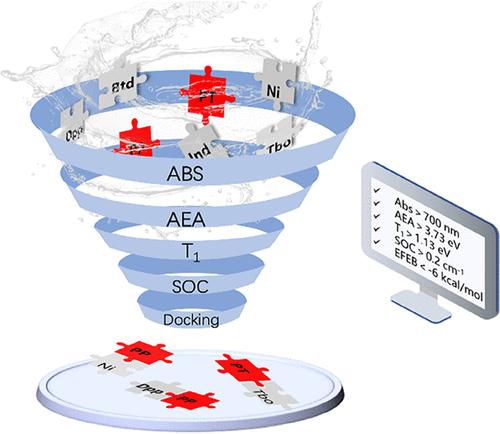
Construction of Photosensitizer Candidates in Photodynamic Therapy: Computer Aided Design, Calculation, and Screening
Thiophene and pyrrole units are extensively utilized in light-responsive materials and have significantly advanced the field of organic photovoltaics (OPV). This progress has inspired our exploration of photosensitizers (PS) for photodynamic therapy (PDT). Currently, traditional PS face limitations in clinical application, including a restricted variety and narrow applicability. Drawing upon molecular design concepts from OPV, we aim to transcend these limitations in PDT. Given the abundance of candidate molecules, effective screening is crucial. Theoretical calculations and electronic structure analyses serve as precise and practical screening methods. In this study, we adopted strategies successfully employed in OPV molecular design, focusing on donor–acceptor (D-A) and acceptor–donor–acceptor (A-D-A) structures. Using density functional theory (DFT) and time-dependent density functional theory (TDDFT), we systematically designed combinations of promising organic fragments. These fragments include polythiophene and polypyrrole-dominated donor structures, paired with five electron acceptors: indene (Ind), diketopyrrole (DPP), naphthalimide (Ni), benzothiazole (Btd), and dithiazolyl diketopyrrole (Tbo). Through meticulous calculations, we obtained electronic structures and spectral properties for all candidate molecules, facilitating an efficient screening process. Our findings highlight that those combinations of polypyrrole-based frameworks with DPP, Ni, and Btd show significant promise for PS applications. Approximately 13% of candidates were selected through comprehensive comparison, markedly reducing molecular design time and experimental costs. This interdisciplinary approach holds potential to pave the way for more targeted and successful PS designs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


